
The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension
Introduction
Coronavirus disease 2019 (COVID-19) is a new respiratory illness caused by a novel coronavirus (designated as SARS-CoV-2), first reported in Wuhan, China, in December 2019. The outbreak of COVID-19 is currently continuously evolving globally, resulting in 221,334 confirmed cases including in health-care workers, worldwide by March 20, 2020 (1). Common symptoms of COVID-19 are a dry cough, a high temperature, fatigue and shortness of breath. In many severe cases, the coronavirus infection caused interstitial pneumonia which may lead to severe acute respiratory syndrome (SARS) and even death (2,3). Thus, the COVID-19 becomes an ongoing public health emergency of international significance.
Recently, a meta-analysis of eligible studies in China revealed the proportion of hypertension in patients with COVID-19 were 17.1%, and the incidences of hypertension was about two folds higher in ICU/severe cases than in their non-ICU/severe counterparts (4). It suggests patients with hypertension may face an increased risk of developing into the severe condition after SARS-CoV-2 infection. The essential hypertension patients are basically treated with blood pressure lowering drugs, mainly including renin-angiotensin system (RAS) blockers, β-blockers, calcium channel blockers (CCBs), diuretics. RAS blockers such as angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) are primarily used to treat hypertension and other cardiovascular diseases to prevent heart/kidney failure or reduce the risk of stroke. Since angiotensin-converting enzyme 2 (ACE2) was identified as the receptor of SARS-CoV-2 as well as SARS- CoV (5), treatment of RAS blockers (ACEIs/ARBs) has attracted more attention. ACE2 is abundantly expressed in airway epithelial cells and induces viral pneumonia during COVID-19 (6). ACE2 is also universally expressed in heart, liver, kidney, blood vessels and other tissues (7,8). Several studies evidenced that ACEIs/ARBs increase ACE2 expression and activity in the heart and kidneys in normotensive or hypertensive rats (9,10), suggesting that ACEIs/ARBs may be a risk factor for fatal Covid-19. However, ACEIs/ARBs prevent the progression of pulmonary complications in vulnerable populations (11), and reduce severe lung injury in certain viral pneumonias (12). Therefore, there is conflicting evidence about that use of ACEIs/ARBs has a positive or negative impact on COVID-19. Under these condition, HFSA/ACC/AHA and ESC just issued statement respectively, they emphasized there is no clinical or scientific evidence supporting harmful effect of ACEIs/ARBs in the context of the pandemic COVID-19 outbreak, recommending continuation of usual ACEIs/ARBs treatment for those hypertension or other cardiovascular diseases patients with COVID-19. They also point out these recommendations will be adjusted as needed to correspond with the latest research (13,14). Therefore, clinical analysis of RAS blockers treatment in patients with COVID is urgently needed.
In the present study, we investigate the clinical and laboratory characteristic of hospitalized COVID-19 patients with hypertension, and compare the difference between ACEIs/ARBs group and other blood pressure lowering drugs group. The study will provide clinical evidence on the impact of ACEIs/ARBs on the clinical course of COVID-19 infection.
Methods
Patients enrollment and data collection
We performed observational registry study in essential hypertension patients with COVID-19 admitted to the Renmin Hospital of Wuhan University (Wuhan, China) from Feb 7 to Mar 03, 2020. Baseline clinical data were collected at admission, and outcome data were collected prospectively by physicians who un-knew the study group. The primary endpoint was the time from illness onset to negative nucleic acid and the secondary endpoints were worsened Chest CT during hospitalization and in-hospital mortality.
Hypertension history and blood pressure medications were self-reported by all the patients. Confirmed COVID-19 cases were defined as those with epidemiological history, consistent with two clinical manifestations, and microbiological evidence [laboratory test for the COVID-19 from the respiratory specimens show positive result by the real-time reverse-transcription-polymerase-chain-reaction (RT-PCR) assay] according to the Novel Coronavirus Pneumonia Diagnosis and Treatment Guideline (5th ed.) (in Chinese) published by the National Health Commission of China (15). Two patients secondary to chronic renal insufficiency were excluded from this study. We collected demographic and clinical data, laboratory parameters, chest CT imaging, management methods, and prognosis through electronic nursing and medical records using standardized data collection form. This study was approved by the institutional ethics board of the Renmin Hospital of Wuhan University (No. WDRY2020-K048). Written informed consent was waived owing to the urgent need to collect data.
Complete blood count, coagulation profile, renal and liver function, creatine kinase, electrolytes, myocardial enzymes, CD4 and CD8 cell counts, C-reactive protein (CRP), D-dimer and procalcitonin were collected routinely on admission. Real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR) assay was used to analyze the SARS-CoV-2 viral nucleic acid from pharyngeal swab specimens of Patients on admission and every 3 days during hospitalization. Detailed protocol was described in previous study (3). Chest radiographs or CT scan were also done for all inpatients on admission and every 5–7 days during hospitalization.
Clinically, illness severity of the COVID-19 patients was classified into mild, moderate, severe, and critically ill according to the Novel Coronavirus Pneumonia Diagnosis and Treatment Guideline (5th ed.) by the National Health Commission of China (15). Radiologically, the area of affected lungs consistent with viral pneumonia in each patient's first chest CT after admission was measured and classified into <30%, 30–50%, and ≥50% of total lung area. During hospitalization, lesion progression in chest CT (worsened chest CT) was judged by 2 independent radiologists.
Discharge criteria includes (15): (I) body temperature returns to normal for more than 3 days; (II) respiratory symptoms improved significantly; (III) lung imaging was significantly improved; (IV) nucleic acid of sputum sample was negative for two consecutive times.
All medical records were collected by cardiologists (Zhongshan Hospital, Fudan University) who has been working in Renmin Hospital of Wuhan University (Wuhan, China) since January 2020 under the coordination of Renmin Hospital of Wuhan University. These records were copied and sent to the data-processing center of Shanghai Institute of Cardiovascular Diseases, Zhongshan Hospital in Shanghai. Experienced physicians and researchers reviewed and analyzed the data.
Statistical analysis
This observational registry study aimed to answer the urgent hypothesis that angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the severity of the currently pandemic of COVID-19, we shift of emphasis from a fixation on sample size to a focus on methodological quality would yield more studies with less bias (16). All data were collected and monitored centrally and summarized with descriptive statistics. Patients who received at least one type of RAS blockers were assigned into Group A (medication including RAS blockers). The rest of patients were assigned into Group B (medication without RAS blockers). Continuous data accorded with normal distribution were expressed as mean ± SD and compared by independent samples t-test, or expressed as median (25th–75th percentile) and compared by Wilcoxon rank sum test. Categorical variables were expressed as number (percentage) and compared by Chi-square test or Fisher exact test, as appropriate. And ordinal categorical variables were compared by Wilcoxon Rank-Sum Test. Statistical analyses were performed with SPSS (v.22.0; SPSS Inc., Chicago, IL, USA) and P value less than 0.05 was considered statistically significant.
Results
Study population
The study population consisted of 50 adult hypertension patients with COVID-19 infections. All these patients including 27 males (54%) and 23 females (46%) were treated with one or multiple blood pressure lowering drugs, including RAS blockers-ACEIs/ARBs (A), β-Blockers (B), calcium channel blockers (C) and diuretics (D). The combinations of these drugs in the cohort are provided in Table 1. Briefly, 10 (50%) and 17 (56.7%) of the Group A and Group B participants were males (P=0.643), and the average age was 52.65±13.12 and 67.77±12.84 years (P=0.000), respectively (Table 2). The underlying diseases including diabetes mellitus, coronary heart disease (CAD), chronic obstructive pulmonary disease (COPD) and anemia in Group A and Group B are listed in Table 2. There was no significant difference between two groups.
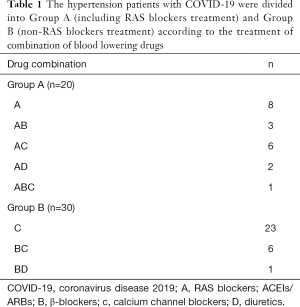
Full table
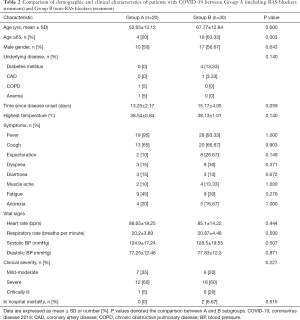
Full table
Comparison of demographic and clinical characteristics
On admission, the SBP was 124.9±17.24 and 128.5±19.55 mmHg, and the DBP was 77.25±12.48 and 77.83±12.3 mmHg in Group A and Group B respectively (Table 2). The data suggest that these blood pressure lowering drugs in Group A and Group B were similarly effective. Time from onset to hospitalization was 13.25±2.17 days in Group A and 15.17±4.05 days in Group B respectively (P=0.059). There was no difference in the highest temperature and the incidence of clinical symptoms including fever, cough, expectoration, shortness of breath et al. between the two groups (Table 2).
The clinical severity was graded as mild-moderate, severe and critically ill. There is no difference in any of grade between Group A and Group B.
Comparison of laboratory and radiologic findings
There was no significant difference in the blood cell test including lymphocyte count and neutrophil count between Group A and Group B (Table 3). Other laboratory findings including C-reactive protein and D-dimer level in Group A showed the similar results compare to those in Group B (Table 3). However, Lower cTnI level (0.01±0.01 vs. 0.1±0.22; P=0.03) and NT-proBNP level (43.39 vs. 263.05; P=0.04) were found in Group A when compared with Group B (Table 3). But the patients with cTnI level more than 0.04 ng/mL or the elevated NT-proBNP level didn’t show significant difference between two groups. In patients over 65 years or under 65 years, cTnI level or NT-proBNP level showed no difference between the two groups (Table 4).
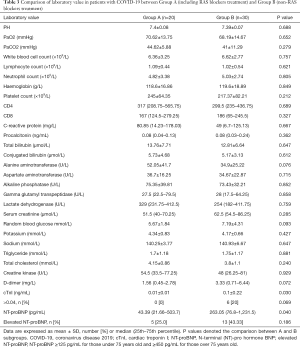
Full table

Full table
All the patients with chest CT scan on admission showed the abnormal results, but these was no difference between the two groups (Table 5).
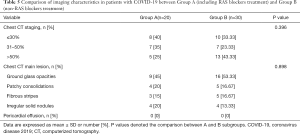
Full table
Comparison of clinical course and short-term outcome
All patients continued to use pre-admission antihypertensive drugs. The management strategy for COVID-19 after admission was analyzed, there was no difference between the two groups in the utilization rate of antiviral therapy, anti-inflammatory therapy and oxygen therapy.
Although there were 3 patients died from pneumonia in Group B, the in-hospital mortality showed no significant difference between two groups (P=0.265). The remaining 47 patients was successfully discharged. The course of disease, namely time from onset to discharge, was 40.7±11.43 days in Group A and 45.38±13.71 days in Group B respectively (P=0.541). Time from illness onset to the first time of negative nucleic acid was also recorded, showing similar results within the two groups (29.3±11.26 vs. 34.48±12.31; P=0.146). There was also no difference regarding the proportion of patients with worsened Chest CT during hospitalization (P=0.450) (Table 6).
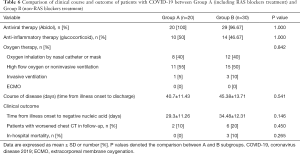
Full table
Discussion
In the present study, we analyzed clinical characteristics of 50 essential hypertension patients with COVID-19, and found there was no obvious difference in clinical course between ACEIs/ARBs medication group and non ACEIs/ARBs medication group.
The essential hypertension patients are basically treated with blood pressure lowering drugs including RAS blockers (ACEIs/ARBs). Currently, the concern about the safety of ACEIs/ARBs treatment arises from the observation that COVID-19 virus binds to a specific enzyme called ACE2 to infect cells (5), and ACE2 expression or activity is increased following treatment with ACEIs and ARBs (9,10). Until now, there is no available clinical data about a differential use of RAS blockers (ACEIs/ARBs) in COVID-19 patients with hypertension.
In the present study, the blood pressure of both groups was under effective control. The age of non-RAS blockers was older than RAS group. CCBs rather than RAS blockers is recommended in elderly Chinese hypertensive population to reduce the risk of stroke, which is the major cardiovascular risk threatening the Chinese hypertensive population (17,18). This can explain the age gap in our study. We observed clinical symptoms including fever, cough, shortness of breath, the vital signs including demographic and clinical characteristics including blood pressure and heart rate, clinical severity on admission. There were no significant difference between RAS blockers group and non-RAS blockers group. We also observed laboratory findings including blood cell counts, blood biochemical indexes, and immunological index (CRP, CD8, CD4). These findings in RAS blockers group showed similar results to those in non-RAS blockers group.
The elevated serum NT-proBNP is related to heart failure and other cardiac problems, and it positively correlates to age of patients (19). We observed NT-proBNP was decreased in RAS blockers group in compared with non-RAS blockers group. But the elevated NT-proBNP (refers to NT-proBNP ≥125 pg/mL for those under 75 years old and ≥450 pg/mL for those over 75 years old) didn’t show any difference between the two groups exclude the factor of age. Serum cTnI, an index for cardiac injury, showed significant lower level in RAS blockers group than that in non-RAS blockers. But there is no difference in cTnI level between the two groups after excluding the age factor. The patients with cTnI more than 0.04 ng/mL or elevated NT-proBNP level had no statistical significance between the two groups. Kennon et al. reported pretreatment with ACEIs reduced troponin release in patients with non-ST elevation acute coronary syndromes (20). However, ACEIs/ARBs are independently associated with postoperative troponin elevation and increased hospital stay in patients with spinal surgery (21). We speculate the effects of ACEIs/ARBs on cardiac troponin are dependent on pathophysiological conditions. According to the clinical data, some patients with COVID-19 developed acute cardiac injury evidenced by the elevated NT-proBNP or cardiac troponin (19). But until now there is no evidence that SARS-2 directly infects cardiomyocytes by ACE2. COVID-19 may affect the cardiovascular system through other mechanisms. COVID-19 mediated- hypoxaemia may be also an important reason of cardiac injury (4). Animal experiments revealed that ACEIs or ARBs protect heart from injury by multiple mechanisms under pressure overload or ischemia. In addition to the upregulation of ACE2 level, these drugs promote the proliferation of endothelial progenitor cells (EPCs), increased periostin expression or blocking TGF-β signaling in heart tissue after myocardial infarction or pressure overload (22-25). Whether ACEIs/ARBs reduces cardiac troponin release or protect cardiac injury in hypertension patients with COVID-19 merits a large population of study.
During hospitalization, these patients received similar therapies including antiviral therapy, anti-inflammatory therapy and oxygen therapy, Course of disease in RAS blockers group was similar to non-RAS blockers group. There is no difference in severe conditions evidenced by patients with worsened CT and in-hospital mortality between the RAS blockers group and non-RAS group. These data provide clinical analysis that RAS blockers doesn’t aggravate the conditions of COVID-19 patients.
Our study has some notable limitations. First, the limited number of cases were studied, the study power was not enough for multivariate regression analysis, a large population study will be required; second, data generation was clinically driven and not systematic. Other cardiac injury indexes need to be examined. Third, we didn’t observe mild cases who were treated at home.
In conclusion, there is no obvious difference between the RAS blockers and non-RAS blockers in clinical features and course of hypertension patients suffering from the COVID-19.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (81870269) and National Key Research and Development Program of China (2018YFC1312703).
Footnote
Provenance and Peer Review: This article was submitted to ATM as a revised version along with the incisive peer review comments after rejection from another esteemed journal. Given the revisions and the wide concern and pressing importance of research relating to COVID-19, the article was managed via the rapid communication pathway and underwent internal review within 72 hours.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional ethics board of the Renmin Hospital of Wuhan University (No. WDRY2020-K048). Written informed consent was waived owing to the urgent need to collect data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Coronavirus disease 2019 (COVID-19). Situation report – 45. Geneva, Switzerland: World Health Organization; March 20, 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020. [Epub ahead of print].
- Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Chen Y, Guo Y, Pan Y. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020;63:457-60. [Crossref] [PubMed]
- Ocaranza MP, Michea L, Chiong M, et al. Recent insights and therapeutic perspectives of angiotensin-(1-9) in the cardiovascular system. Clin Sci (Lond) 2014;127:549-57. [Crossref] [PubMed]
- Keidar S, Kaplan M, Gamliel-Lazarovich A. ACE2 of the heart: From angiotensin I to angiotensin (1-7). Cardiovasc Res 2007;73:463-9. [Crossref] [PubMed]
- Mendoza-Torres E, Oyarzún A, Mondaca-Ruff D, et al. ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther Adv Cardiovasc Dis 2015;9:217-37. [Crossref] [PubMed]
- Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111:2605-10. [Crossref] [PubMed]
- Soto M, Bang SI, McCombs J. Renin Angiotensin system-modifying therapies are associated with improved pulmonary health. Clin Diabetes Endocrinol 2017;3:6. [Crossref] [PubMed]
- Caldeira D, Alarcão J, Vaz-Carneiro A, et al. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta-analysis. BMJ 2012;345:e4260. [Crossref] [PubMed]
- Bozkurt B, Kovacs R, Harrington B. HFSA/ACC/AHA statement addresses concerns re:using RAAS antagonists in COVID-19. March 17, 2020. Available online: https://professional.heart.org/professional/ScienceNews/UCM_505836_HFSAACCAHA-statement-addresses-concerns-re-using-RAAS-antagonists-in-COVID-19.jsp
- ESH Statement on COVID-19: Statement of the European Society of Hypertension (ESH) on hypertension, Renin Angiotensin System blockers and COVID-19. March 12, 2020. Available online: https://www.eshonline.org/
- General Office of National Health Commission. Diagnosis and treatment of novel coronavirus pneumonia (5th edition, 2020) 2020:1-12.
- Kenneth Schulz, David Grimes. Essential Concepts in Clinical Research - 2nd Edition Essential Concepts in Clinical Research: Randomised Controlled Trials and Observational Epidemiology (2nd ed.). Elsevier, 24th September 2018, ISBN: 9780702073946.
- Joint Committee for Guideline. R. 2018 Chinese Guidelines for Prevention and Treatment of Hypertension-A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol 2019;16:182-241. [PubMed]
- Wang JG, Kario K, Lau T, et al. Use of dihydropyridine calcium channel blockers in the management of hypertension in Eastern Asians: a scientific statement from the Asian Pacific Heart Association. Hypertens Res 2011;34:423-30. [Crossref] [PubMed]
- Dzudie A, Dzekem BS, Kengne AP. NT-pro BNP and plasma-soluble ST2 as promising biomarkers for hypertension, hypertensive heart disease and heart failure in sub-Saharan Africa. Cardiovasc J Afr 2017;28:406-7. [PubMed]
- Kennon S, Barakat K, Hitman GA, et al. Angiotensin-converting enzyme inhibition is associated with reduced troponin release in non-ST-elevation acute coronary syndromes. J Am Coll Cardiol 2001;38:724-8. [Crossref] [PubMed]
- McClendon J Jr, Smith TR, Thompson SE, et al. Renin-angiotensin system inhibitors and troponin elevation in spinal surgery. J Clin Neurosci 2014;21:1133-40. [Crossref] [PubMed]
- Wang X, Ye Y, Gong H, et al. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang(1-7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J Mol Cell Cardiol 2016;97:180-90. [Crossref] [PubMed]
- Wang Q, Chen Z, Huang X, et al. Olmesartan attenuates pressure-overload- or post-infarction-induced cardiac remodeling in mice. Oncotarget 2017;9:24601-18. [PubMed]
- Müller P, Kazakov A, Semenov A, et al. Ramipril and telmisartan exhibit differential effects in cardiac pressure overload-induced hypertrophy without an additional benefit of the combination of both drugs. J Cardiovasc Pharmacol Ther 2013;18:87-93. [Crossref] [PubMed]
- Yang Y, Cui Y, Peng DQ. ARB may be superior to ACEI on treatment of Marfan's syndrome by blocking TGF-β mediated activation of ERK. Int J Cardiol 2012;155:482-3. [Crossref] [PubMed]


