Relationship between body mass index and the risk of periprosthetic joint infection after primary total hip arthroplasty and total knee arthroplasty
Introduction
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are generally regarded as two of the most commonly done and highly successful surgical interventions performed annually to treat end-stage joint disease (1,2). Increasing levels of obesity and population aging, the volume of total joint arthroplasty (TJA) in the United States has increased steadily during the past several decades and is predicted to increase continuously (3,4). The demand for primary THA is projected to grow 71%, to 635,000 procedures, by 2030 and the demand for primary TKA is projected to grow 85%, to 1.26 million procedures, by 2030 in United States (5). A similar pattern of historical increase in the incidence of joint arthroplasty has been reported by many worldwide joint registries, such as New Zealand (6), United Kingdom (7) and Australia (8). With the increasing burden of joint replacement surgery, it is required to ensure that demand can be met and desired outcomes can be achieved.
Periprosthetic joint infection (PJI), also referred to as prosthetic joint infection, is defined as an infection involving the artificial joint prosthesis and adjacent tissue (9). It is a devastating complication of arthroplasty and causes pain, loss of function, systemic inflammatory, and even death, which will lead to a tremendous burden to patients and health-care systems worldwide. As the volume of TJA procedures constantly increasing in the United States using the Nationwide Inpatient Sample, the PJI incidence rate increased from 1.99% in 2001 to 2.18% in 2009 for THA, and from 2.05% in 2001 to 2.18% in 2009 for TKA, respectively (10). More significantly, PJI is the most common cause for revision TKA (25.2%), and is the third most common cause for revision THA (14.8%), following instability/dislocation (22.5%) and mechanical loosening (19.7%) (11,12). Recognition and optimization of any modifiable risk factors before joint arthroplasty are central to the reduction of the prevalence of PJI.
The PJI is attributed to multiple factors, including patient, surgical, and health-care factors. High body mass index (BMI), as a modifiable factor, has been recognized for its association with increased risk of PJI in many but not all previous studies (9,13,14). However, the relationship between BMI and the incidence of PJI remains controversial. After a review of 9,245 patients who underwent primary TJA, Pulido et al. found that morbid obesity (BMI >40 kg/m2) was an independent predictor of infection (13). Berbari et al. reported that a low BMI of <25 kg/m2 was associated with an increased risk of PJI in patients undergoing THA or TKA (15). Meanwhile, Shohat et al. documented that the risk for PJI increases gradually throughout the full range of BMI, but no threshold exists (16). To better understand the impact of increasing BMI on PJI after TJA, we conducted this dose-response analysis to assess the dose-response relationship between BMI and the risk of PJI in patients undergoing primary THA or TKA.
Methods
The present study was conducted and reported following the guidelines for Meta-Analyses and Systematic reviews for Observational Studies and the PRISMA guidelines (17,18).
Search strategy
The systematic literature search without restrictions of language was performed in three databases (PubMed, Embase, Cochrane Library) from their inception to August 17, 2019. The following search terms were used: “total hip arthroplasty”, “total knee arthroplasty”, “body mass index”, “periprosthetic joint infection” and their variants. Details of the search strategy are available in Tables S1,S2,S3. Also, the reference list of retrieved studies and relevant reviews were carefully checked for any potential inclusion.

Full table

Full table

Full table
Study selection
After the removal of duplicates, two authors (Junlong Zhong and Bin Wang) independently screened the titles and abstracts of potentially relevant studies, and full text of all relevant studies was obtained for systematic assessment against the inclusion criteria. Studies were eligible for inclusion if they met the following criteria: (I) a case-control study or a cohort study design; (II) patients undergoing primary THA or primary TKA; (III) overweight, obesity, and BMI were the exposures of interest; (IV) PJI was the outcome of interest [diagnostic criteria of PJI as reported by Parvizi et al. (19)]; and (V) investigated the association between BMI and PJI, and reported appropriate estimates such as the hazard ratio (HR), relative risk (RR) or odds ratio (OR) and the corresponding 95% confidence interval (CI). Case reports, editorials, letters, comments, reviews, conference abstracts, and studies that did not report sufficient data were excluded. If the same participants were overlapping reported, we retained only the most recent one or the most informative one to avoid duplication of information.
Data extraction
Data were extracted independently by two authors (Junlong Zhong and Yufeng Chen) and were checked for accuracy by another author (Bin Wang). The following variables were extracted from each included studies: first author, year of publication, country, research databases, study period, study design, number of participants, participant’s mean age, type of surgery, number of PJI, type of PJI, duration of follow-up, BMI categories (at least three BMI categories), number of participants in each category, adjusted HRs, RRs or ORs with the corresponding 95% CIs, and other interesting items. All the extracted data were entered into a predetermined Excel (Microsoft Corporation, USA) file.
Quality assessment of studies
The quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS), which included 8 items and allowed a maximum of nine stars (20). We assigned scores of 0 to 3, 4 to 6, and 7 to 9 for the low, moderate, and high quality of studies, respectively.
Statistical analysis
The ORs and HRs were directly considered as equivalent to the RRs because of the incidence of PJI is rare (21). The RRs were calculated assuming the minimum BMI category as the reference category. The median or mean BMI in each category was assigned to each corresponding adjusted RRs and 95% CIs. If the median or mean BMI per category was not reported in one study, we defined the midpoint of the upper and lower boundaries of each BMI category as the average. When the highest or lowest category was open-ended, we assumed that the width of the interval to be equal to the adjacent category. Beyond that, we considered mean BMI is 18 kg/m2 for BMI ≤18.5 kg/m2 and 41 kg/m2 for BMI ≥40 kg/m2. We used the method proposed by Greenland et al. and Orsini et al. to conduct a two-stage random-effects dose-response analysis, which required cases of PJI, doses of BMI and corresponding adjusted RRs and 95% CIs in at least three BMI categories (22,23). Furthermore, other potential risk factors for PJI were pooled using a random or fixed effects model as a secondary outcome.
The degree of heterogeneity among included studies was assessed using the Cochran’s Q test and quantified by calculating the I2 statistic (24,25). An I2>50% or P<0.1 indicated significant heterogeneity, and then a random-effects model was used; otherwise, a fixed-effects model was used. To perform sensitivity analysis, one study was omitted in each turn and the remaining studies were analyzed to explore the impact of the individual study on the overall risk of PJI. Publication bias was also evaluated using Begg’s test and Egger’s test (26,27). If any publication bias was detected, it was checked via the “trim and fill method” (28) for estimating the impact of these missing studies on overall effect size. A P<0.05 was considered statistically significant. All statistical analyses were performed using Stata 14.0 (Stata Corporation, College Station, TX, USA).
Results
Study selection
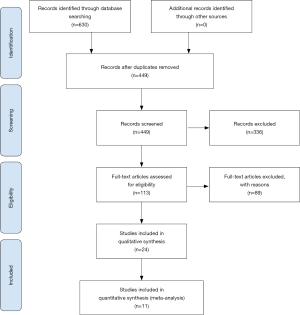
The flow diagram of the study selection process is presented in Figure 1. A total of 630 records were identified through the aforementioned databases from their inception to August 17, 2019. After the exclusion of duplicates and irrelevant records based on titles and abstracts, the remaining 113 studies were further assessed for detailed full-text analysis. After reviewing the full text in detail, 24 studies met the inclusion criteria and were included in the qualitative synthesis. Thirteen studies that did not report available data for analysis were excluded from the quantitative meta-analysis. Finally, 11 studies were eligible for inclusion in the present analysis.
Study characteristics
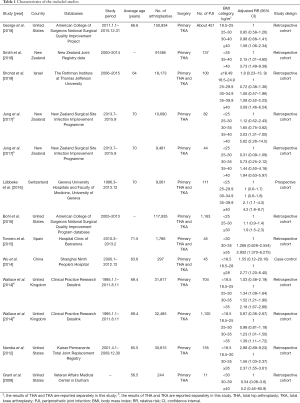
The characteristics of the included studies are shown in Table 1. A total of 11 observational studies (including 10 cohort studies and 1 case-control study) were included in the present analysis (16,29-38). These 11 studies were published between 2008 and 2018, and were predominantly from North America (n=4), followed by Europe (n=3), Oceania (n=2) and Asia (n=2). Regarding the site of arthroplasty, one study (29) and three studies (30-32) only reported outcomes in knee and hip, respectively, while seven studies (16,33-38) reported outcomes in both sites. Of the seven studies, two studies (33,38) reported outcomes in knee and hip separately, and five studies (16,34-37) reported combining data of both sites. A total of 505,303 participants undergoing primary THA or TKA, which involved 4,148 cases of PJI, were included among these 11 studies. The average age of the participants in each study varied from 56.5 to 71.5 years. The detailed score of NOS of each study is summarized in Table 2. The average score of the NOS was 6.9, and the score for each study was 6 or above, indicating that all included studies were of moderate or high quality.
Full table

Full table
Primary outcome: dose-response analysis
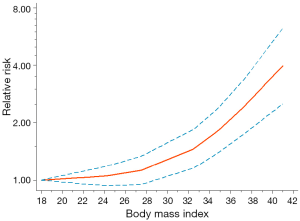
A total of 11 studies were included for dose-response analysis. We observed a significant non-linear dose-response relationship between BMI and the risk of PJI in patients undergoing primary THA or TKA (Pnon-linearity <0.001). Figure 2 showed the impact of BMI as a continuous variable on the risk of PJI. The J-shaped dose-response curve indicated that the increase of BMI accompanied with an accelerated increase of postoperative PJI rate in patients undergoing primary THA or TKA, especially when the BMI was greater than 24 kg/m2 (reaching the upper limit of normal BMI).
Secondary outcomes
Furthermore, we conducted a meta-analysis to identify other risk factors for PJI, including gender (male vs. female), American Society of Anesthesiologists (ASA) score (ASA score ≥3 vs. ASA score <3), surgical site (TKA vs. THA), and comorbidities (including lung disease, diabetes and hypertension).
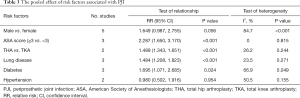
Five studies (30,31,33,36,37) including a total of 144,321 participants (PJI =559, and non-PJI =143,762) reported gender as a potential risk factor for PJI. The pooled result showed no significant difference in the risk of PJI between the male patient (RR, 1.649; 95% CI, 0.987–2.755; P=0.056) and the female patient. There was significant heterogeneity among these studies (I2=84.7%, Pheterogeneity<0.001).
Three studies (30,31,33) including a total of 55,165 participants (PJI =225, and non-PJI =54,940) reported ASA score as a potential risk factor for PJI. The pooled result showed that the higher ASA score (ASA score ≥3) was positively associated with the higher incidence of PJI (RR, 2.287; 95% CI, 1.650–3.170; P<0.001). No significant heterogeneity was found among these studies (I2=0, Pheterogeneity=0.815).
Two studies (35,37) including a total of 118,232 participants (PJI =1,208, and non-PJI =117,024) reported surgical site (TKA vs. THA) as a potential risk factor for PJI. The pooled result showed that the patients following THA were more likely to suffer from PJI than the patients following TKA (RR, 1.489; 95% CI, 1.343–1.651; P<0.001). No significant heterogeneity was found among these studies (I2=26.2, Pheterogeneity=0.244).
As for the comorbidities, the pooled result revealed that the patients suffered from lung disease (RR, 1.484; 95% CI, 1.208–1.823; P<0.001) or diabetes (RR, 1.695; 95% CI, 1.071–2.685; P=0.024) were significantly associated with the increased incidence of PJI, while the patients who suffered from hypertension (RR, 0.980; 95% CI, 0.502–1.916; P=0.954) were not significantly associated with the increased incidence of PJI. The results of heterogeneity analysis were shown in Table 3.
Full table
Sensitivity analysis and publication bias
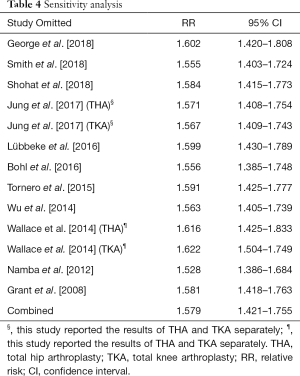
To evaluate the influence of each study on the combined effect, we conducted a sensitivity analysis. Exclusion of any individual study, we observed no significant change of the relationship between obesity (compared with non-obesity) and risk of PJI (Table 4). The Begg’s test and Egger’s test (PBegg=0.300; PEgger=0.099) showed that there was no significant publication bias in the present study.
Full table
Discussion
Causes of PJI are multifactorial and can be broadly classified into patient-related factors, surgery-related factors, and hospital-related factors. In the present study, we identified a significant non-linear relationship between BMI and the risk of PJI in patients undergoing primary THA or TKA. Also, our study revealed that higher ASA score (ASA score ≥3), lung disease, and diabetes were significant risk factors for PJI, and the patients following THA were more likely to suffer from PJI than the patients following TKA. However, we did not observe significant associations between hypertension and gender and the risk of PJI.
In the present study, we evaluated patient-related risk factors for PJI, including gender, BMI, ASA score, and some comorbidities. We found that the RR of PJI increased with the increase of BMI, and dramatically increased in obese patients. As documented in previous literature, the increased risks of PJI in patients with higher BMI are mainly attributed to thicker adipose tissue layer (large potential dead space that enhanced the risk of hematoma), additional comorbidities (such as diabetes mellitus, lung disease), longer operative time, and even increased allogeneic blood transfusions (39,40). Also, obese patients have impaired tissue antibiotic penetration, and an under-dosing of tissue concentrations leads to an increased risk of infection (41). Furthermore, some studies suggested that obesity is a pro-inflammatory state associated with a low-grade inflammatory response, and may affect the postoperative immune response and increase the risk of surgical site infection (SSI) (42,43). Previous studies reported that males had a significantly increased risk for PJI than females for both TKA and THA, which may be attributed to some potential confounding variables, such as smoking and alcohol consumption (31,44). After controlling for possible confounders, our results revealed that male was not an independent risk factor for PJI in patients following primary THA or TKA with high heterogeneity (RR, 1.649; P=0.056; I2=84.8%; Pheterogeneity<0.001). Meanwhile, our results revealed that patients with diabetes, lung disease or higher ASA score (ASA score ≥3) had an increased risk of PJI, but hypertension was not a risk factor for PJI. It is reported that patients with diabetes, especially those with insulin-dependent diabetes, exhibited significantly increased odds of postoperative infection, due to hyperglycemia may impair the immune system and provide a favorable environment for certain bacterial reproduction (45,46). Pathogenic bacteria located in lung lesions, mainly including pneumonia and chronic bronchitis, can spread through blood circulation or lymphatic circulation to the surgical site and become a potential source of PJI. The higher ASA score for patients indicates the poor physical condition and serious multiple comorbidities, such as obesity, cardiopulmonary diseases, immune diseases and metabolic diseases, which may contribute to increasing overall infection risk (47). Unfortunately, other patient-related risk factors for PJI after primary THA and TKA, such as age, smoking, alcohol consumption and other basic diseases, were not analyzed due to insufficient data in the present study.
Some potential confounding variables, such as surgery-related factors and hospital-related factors, were not analyzed due to lack of available data in this study. To recognize the influence of these related factors comprehensively, we had a discussion about that based on published literature and data concerned. Among the surgery-related factors, anesthetic management, operative procedure, operative time, drain usage and blood transfusion were possibly associated with PJI following TJA. A retrospective study found that patients receiving primary THA or TKA under general anesthesia were associated with a higher risk of PJI compared with epidural or spinal anesthesia (48). Prolonged operative time was also associated with a higher risk of PJI following TJA, which may result from increased potential wound contamination, increased soft tissue damage, increased blood loss, and even perioperative transfusion. The risk of PJI significantly increased in primary THA in which operative time lasted for 120 minutes or more (49). Also, Kurtz et al. reported that TKA operative time lasted more than 210 minutes was associated with an increased risk of infection in comparison with those less than 120 minutes (50), and Namba et al. found that each 15-minute increase in operative time was associated with a 9% increased risk of PJI following TKA (51). Significantly, postoperative wound drainage reduced the incidence of hematoma formation and subsequently decreased the risk of infection after TJA. However, persistent wound drainage more than 48 hours has been identified as a risk factor for PJI (52). Patel et al. reported that every additional day of prolonged wound drainage increased the risk of wound infection by 42% in THA and by 29% in TKA (53). In addition, although the use of blood products during the perioperative period is a valuable means for the treatment of anemia, the immunomodulating response of allogeneic blood transfusion may be the cause of the increased risk of PJI (54). The impact of surgical approach or prosthesis selection on infection is needed to be confirmed in further research.
Furthermore, the incidence of PJI following TJA was decreasing with the surgeon’s or hospital’s arthroplasty volume increased. Generally, increased arthroplasty volume has been associated with decreased length of stay, resulting in decreased exposure to nosocomial organisms (13). It is also possible that the medical staff in a high-volume institution had more experience in recognizing the early signs and symptoms of developing infection and in taking efficient measures to prevent infection (55,56). In previous studies, it was reported that laminar flow ventilation in the operating room was identified as a risk factor for PJI (30,57). It may be that improper positioning of surgical personnel and lower intraoperative tissue temperatures in the surgical wound increased the risk of infection (58).
Some limitations of this study should be highlighted. First, most of the included studies were observational and retrospective and therefore limited the ability to control for confounding variables, which may have a certain impact on the credibility of the results. Second, the race of patients, primary disease, surgical indication, and follow-up duration varied among these studies, which might result in substantial heterogeneity. Third, due to the limited number of studies available, it was impossible to estimate the effects of all potential risk factors (such as basic diseases, surgery-related factors and hospital-related factors) and to perform valuable subgroup analysis. Further high-quality studies are warranted to comprehensively clarify the risk factors for PJI after primary THA and TKA.
Conclusions
In conclusion, the J-shaped non-linear relationship between BMI and the risk of PJI demonstrated that increased BMI was associated with an increased risk for PJI in patients undergoing primary THA or TKA. Patients following THA were more likely to suffer from PJI than patients following TKA. Besides, ASA score ≥3, lung disease and diabetes were identified as significant risk factors for PJI, but gender and hypertension were not recognized as risk factors for PJI. Further research is warranted to confirm these findings and to develop effective prevention.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 81572174; 81772384).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.112). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferguson RJ, Palmer AJ, Taylor A, et al. Hip replacement. Lancet 2018;392:1662-71. [Crossref] [PubMed]
- Price AJ, Alvand A, Troelsen A, et al. Knee replacement. Lancet 2018;392:1672-82. [Crossref] [PubMed]
- Kurtz SM, Ong KL, Schmier J, et al. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty 2009;24:195-203. [Crossref] [PubMed]
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780-5. [Crossref] [PubMed]
- Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am 2018;100:1455-60. [Crossref] [PubMed]
- Hooper G, Lee AJJ, Rothwell A, et al. Current trends and projections in the utilisation rates of hip and knee replacement in New Zealand from 2001 to 2026. N Z Med J 2014;127:82-93. [PubMed]
- Culliford D, Maskell J, Judge A, et al. Future projections of total hip and knee arthroplasty in the UK: Results from the UK Clinical Practice Research Datalink. Osteoarthritis Cartilage 2015;23:594-600. [Crossref] [PubMed]
- Inacio MC, Graves SE, Pratt NL, et al. Increase in total joint arthroplasty projected from 2014 to 2046 in Australia: A conservative local model with international implications. Clin Orthop Relat Res 2017;475:2130-7. [Crossref] [PubMed]
- Kapadia BH, Berg RA, Daley JA, et al. Periprosthetic joint infection. Lancet 2016;387:386-94. [Crossref] [PubMed]
- Kurtz SM, Lau E, Watson H, et al. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 2012;27:61-5.e1. [Crossref] [PubMed]
- Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the united states. J Bone Joint Surg Am 2009;91:128-33. [Crossref] [PubMed]
- Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the united states. Clin Orthop Relat Res 2010;468:45-51. [Crossref] [PubMed]
- Pulido L, Ghanem E, Joshi A, et al. Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710-5. [Crossref] [PubMed]
- Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 1998;27:1247-54. [Crossref] [PubMed]
- Berbari EF, Osmon DR, Lahr B, et al. The Mayo prosthetic joint infection risk score: Implication for surgical site infection reporting and risk stratification. Infect Control Hosp Epidemiol 2012;33:774-81. [Crossref] [PubMed]
- Shohat N, Fleischman A, Tarabichi M, et al. Weighing in on body mass index and infection after total joint arthroplasty: Is there evidence for a body mass index threshold? Clin Orthop Relat Res 2018;476:1964-9. [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. [Crossref] [PubMed]
- Parvizi J, Tan TL, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 2018;33:1309-14.e2. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1-30. [Crossref] [PubMed]
- Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301-9. [Crossref] [PubMed]
- Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006;6:40-57. [Crossref]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. [Crossref] [PubMed]
- George J, Piuzzi NS, Ng M, et al. Association between body mass index and thirty-day complications after total knee arthroplasty. J Arthroplasty 2018;33:865-71. [Crossref] [PubMed]
- Smith JO, Frampton CMA, Hooper GJ, et al. The impact of patient and surgical factors on the rate of postoperative infection after total hip arthroplasty-a New Zealand Joint Registry study. J Arthroplasty 2018;33:1884-90. [Crossref] [PubMed]
- Namba RS, Inacio MC, Paxton EW. Risk factors associated with surgical site infection in 30,491 primary total hip replacements. J Bone Joint Surg Br 2012;94:1330-8. [Crossref] [PubMed]
- Grant JA, Viens N, Bolognesi MP, et al. Two-year outcomes in primary THA in obese male veterans administration medical center patients. Rheumatol Int 2008;28:1105-9. [Crossref] [PubMed]
- Jung P, Morris AJ, Roberts SA, et al. BMI is a key risk factor for early periprosthetic joint infection following total hip and knee arthroplasty. N Z Med J 2017;130:24-34. [PubMed]
- Lübbeke A, Zingg M, Vu D, et al. Body mass and weight thresholds for increased prosthetic joint infection rates after primary total joint arthroplasty. Acta Orthop 2016;87:132-8. [Crossref] [PubMed]
- Bohl DD, Sershon RA, Fillingham YA, et al. Incidence, risk factors, and sources of sepsis following total joint arthroplasty. J Arthroplasty 2016;31:2875-9.e2. [Crossref] [PubMed]
- Tornero E, Garcí-Ramiro S, Martínez-Pastor JC, et al. Prophylaxis with teicoplanin and cefuroxime reduces the rate of prosthetic joint infection after primary arthroplasty. Antimicrob Agents Chemother 2015;59:831-7. [Crossref] [PubMed]
- Wu C, Qu X, Liu F, et al. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One 2014;9:e95300. [Crossref] [PubMed]
- Wallace G, Judge A, Prieto-Alhambra D, et al. The effect of body mass index on the risk of post-operative complications during the 6 months following total hip replacement or total knee replacement surgery. Osteoarthritis Cartilage 2014;22:918-27. [Crossref] [PubMed]
- Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res 2009;467:1577-81. [Crossref] [PubMed]
- Wagner ER, Kamath AF, Fruth K, et al. Effect of body mass index on reoperation and complications after total knee arthroplasty. J Bone Joint Surg Am 2016;98:2052-60. [Crossref] [PubMed]
- Toma O, Suntrup P, Stefanescu A, et al. Pharmacokinetics and tissue penetration of cefoxitin in obesity: implications for risk of surgical site infection. Anesth Analg 2011;113:730-7. [Crossref] [PubMed]
- Ghanim H, Aljada A, Hofmeyer D, et al. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 2004;110:1564-71. [Crossref] [PubMed]
- Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc 2012;71:298-306. [Crossref] [PubMed]
- Tayton ER, Frampton C, Hooper GJ, et al. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64,566 joints from the New Zealand Joint Registry. Bone Joint J 2016;98-B:334-40. [Crossref] [PubMed]
- Marchant MH, Viens NA, Cook C, et al. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am 2009;91:1621-9. [Crossref] [PubMed]
- Frydrych LM, Bian G, O’Lone DE, et al. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol 2018;104:525-34. [Crossref] [PubMed]
- Hackett NJ, De Oliveira GS, Jain UK, et al. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg 2015;18:184-90. [Crossref] [PubMed]
- Chang CC, Lin HC, Lin HW, et al. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology 2010;113:279-84. [Crossref] [PubMed]
- Ridgeway S, Wilson J, Charlet A, et al. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br 2005;87:844-50. [Crossref] [PubMed]
- Kurtz SM, Ong KL, Lau E, et al. Prosthetic joint infection risk after TKA in the medicare population. Clin Orthop Relat Res 2010;468:52-6. [Crossref] [PubMed]
- Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am 2013;95:775-82. [Crossref] [PubMed]
- Jaberi FM, Parvizi J, Haytmanek CT, et al. Procrastination of wound drainage and malnutrition affect the outcome of joint arthroplasty. Clin Orthop Relat Res 2008;466:1368-71. [Crossref] [PubMed]
- Patel VP, Walsh M, Sehgal B, et al. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am 2007;89:33-8. [Crossref] [PubMed]
- Raghavan M, Marik PE. Anemia, allogenic blood transfusion, and immunomodulation in the critically ill. Chest 2005;127:295-307. [Crossref] [PubMed]
- Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am 2001;83:1622-9. [Crossref] [PubMed]
- Katz JN, Barrett J, Mahomed NN, et al. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am 2004;86:1909-16. [Crossref] [PubMed]
- Gastmeier P, Breier AC, Brandt C. Influence of laminar airflow on prosthetic joint infections: A systematic review. J Hosp Infect 2012;81:73-8. [Crossref] [PubMed]
- Brandt C, Hott U, Sohr D, et al. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg 2008;248:695-700. [Crossref] [PubMed]