Translational assessment of mitochondrial dysfunction of pancreatic cancer from in vitro gene microarray and animal efficacy studies, to early clinical studies, via the novel tumor-specific anti-mitochondrial agent, CPI-613
Introduction
Pancreatic cancers
Pancreatic cancer is the fourth leading cause of cancer death, and its prognosis is grim: 5-year survival rate being 6% (1). Due to a lack of treatment options, Gemzar® (Gemcitabine) became the reference regimen for advanced pancreatic cancer for many years, even though Gemzar® offers only marginal improvement in overall survival (OS) (5.6 vs. 4.4 months, Gemzar®vs. fluorouracil) (2). The combination of Gemzar® with a variety of cytotoxic and targeted agents has generally shown no significant survival advantage as compared with Gemzar® alone (2). Some clinical studies have suggested a benefit with Gemzar®-based cytotoxic combinations in patients with good performance status (2). However, Gemzar®-based cytotoxic combinations are very toxic and patients experience poor quality of life during treatment. The most promising results reported to date is FOLFIRINOX (a 4-drug combination of 5-FU, leucovorin, irinotecan and oxaliplatin) (3), and the Abraxane® (paclitaxel protein-bound particles for injectable suspension) plus Gemzar® combination (4), which provide a median OS of 12.2 and 8.5 months, respectively, in metastatic pancreatic cancer patients. However, these drugs have high toxicity and only patients with good performance status are eligible to receive these regimens. Regardless of the treatment, patients with good performance status have longer survival on average than those with low performance status. Also, although these drugs significantly improve patient survival, the median OS of approximately 12 months in patients with good performance status is still unsatisfactory. A safer and more effective therapy for advanced pancreatic cancer is currently an active area of research and development.
Assessment of genetic alterations to predict chemotherapeutic response
One of the major reasons for a lack of significant progress in developing new therapies for pancreatic cancer is due to the complex genetic alterations associated with its pathogenesis and progression, as chemoresistance of tumor cells can be due to mutations in oncogenes, loss of tumor suppressors, as well as dysregulation of genes involved in DNA repair, cell cycle, cell proliferation, signal transduction, angiogenesis and apoptosis (5,6). Gene alterations occur in many genes, and the four high-frequency pancreatic cancer driver genes are KRAS (>95%), p16/CDKN2A (95%), TP53 (50-75%) and DPC4/SMAD4 (55%) (7-9). Inactivation of DPC4/SMAD4 has been shown to be associated with poor prognosis and wide metastatic phenotypes (10,11).
Assessment of genetic alterations to predict chemotherapeutic response is important in the treatment of cancer. Numerous studies have addressed chemosensitivity testing in a variety of malignancies, only few studies are related to pancreatic cancer (12-14). A recent study investigated the chemosensitivity of a broad panel of genetically defined pancreatic cancers to seven chemotherapeutic drugs with distinct mechanisms of cytotoxicity [gemcitabine, docetaxel, mitomycin C, irinotecan, cisplatin, a Parp inhibitor (KU0058948), and a negative control drug (artemisinin)] using a panel of genetically defined human pancreatic cancer cells. Preliminary data demonstrate correlation of genetic backgrounds with in vitro chemosensitivity (15).
CPI-613, a novel tumor-specific anti-mitochondrial agent
CPI-613 is the first of a novel class of anti-cancer agents, and it is a tumor-specific anti-mitochondrial energy metabolism agent (16-18). CPI-613 targets multiple altered and dysregulated form of enzymes found in tumor cells, and treatment of tumor cells with CPI-613 causes altered energy metabolism and altered redox process, leading to apoptosis, necrosis and autophagy (16-18). The multi-target approaches of CPI-613 include (I) inhibition of tumor cell pyruvate dehydrogenase complex (PDC) through activation of pyruvate dehydrogenase kinases (PDKs) leading to inactivating phosphorylation of the E1α-subunit of PDC; and (II) inhibition of alpha-ketoglutarate dehydrogenase (KGDH, a major tricarboxylic acid cycle mitochondrial enzyme). The anti-tumor activity of CPI-613 in cell culture, various animal tumor models, and clinical trials against diverse cancers have been reported (16-24). Additionally, CPI-613 is generally well tolerated at doses up to 3,000 mg/m2, and can be used in patients with poor performance status and of advanced age, according to a Phase I dose-escalation trial in patients with solid tumors (22-24) and hematologic malignancies (19-21). Due to the safety profile and anti-cancer activities, CPI-613 is currently being investigated in numerous clinical trials as monotherapy, and in combination with other anti-cancer agents, for various solid tumors and hematological malignancies.
Study rationale and objective
Via genetic alteration, malignant transformation and proliferation have been shown to be associated with extensive alterations in mitochondrial energy metabolism in tumor cells (25,26). Pancreatic adenocarcinoma provides fertile ground for altered energy metabolism. Thus, inhibition of altered form of mitochondrial energy metabolism in pancreatic tumor cells may be an effective therapy for pancreatic cancers. The current manuscript describes the results of translational assessment of mitochondrial dysfunction of pancreatic cancer from in vitro gene microarray and animal efficacy studies, to early clinical studies, via the novel tumor-specific anti-mitochondrial agent, CPI-613.
Materials and methods
Test articles
CPI-613 (Cornerstone Pharmaceuticals, Inc., USA) was diluted with 5% Dextrose in Water (D5W), where Gemcitabine (Gemzar, Eli Lilly and Company) was diluted with 0.9% NaCl, immediately before dosing to provide a dose volume of ~80 mL/kg for both drugs in animal studies. Control vehicle was D5W for the animal studies.
Cell culture
BxPC-3 cells, a human pancreatic tumor cell line originally obtained from American Type Cell Culture (ATCC) (Manassas, VA, USA), were used. These tumor cells had been tested negative for viral contamination using the Mouse Antibody Production (MAP) test, performed by Charles River Labs Molecular Division, upon the receipt of the tumor cells from ATCC. The tumor cells were maintained at 37 °C in a humidified 5% CO2 atmosphere in T225 tissue culture flasks containing 50 mL of Roswell Park Memorial Institute (RPMI)-1640 solution (Mediatech, Inc, Manassas, VA, USA) with 10% Fetal Bovine Serum (FBS) (Mediatech, Inc, Manassas, VA, USA). Cells were split at a ratio of 1:3 every 4-5 days by trypsinization and resuspended in fresh medium in a new flask. Cells were harvested for experiments in the same way at 70-90% confluence.
Non-transformed NIH-3T3 mouse fibroblast cells were obtained from ATCC (Manassas, VA, USA). The NIH-3T3 cells were cultured as above in Dulbecco’s Modified Eagle’s Medium solution (ATCC, Manassas, VA, USA) supplemented with 10% FBS (Mediatech, Inc, Manassas, VA, USA).
Profiling genes regulated by CPI-613 using microarray technique
BxPC-3 human pancreatic tumor cells were treated with 50 µM CPI-613 or sham treated (i.e., vehicle), whereas the non-transformed NIH-3T3 mouse fibroblast cells were treated with 100 µM CPI-613 or sham treatment (i.e., vehicle), for 6 hours. The duration of treatment with CPI-613 was based on a time course experiment which measured the time taken to induce death in 10-15% of the cells. At the end of the 6-hour treatment period, live cells were selected based on Trypan blue exclusion and quantified using a hemocytometer. RNA was isolated from 5×103 live cells from each treatment group using the RNeasy micro kit (Qiagen, Valencia, CA, Spain) according to the manufacturer’s specifications. The concentration and quality of isolated RNA were determined by measuring the absorbance at 260 and 280 nm, respectively, using a Bio Rad SmartSpec 3000 spectrophotometer. Gene microarray expression profiling was then performed by SABioscience Service Core Facility (Frederick, MD) using the HumanHT-12 v4 Expression BeadChip Kit (Illumia, San Diego, CA, USA). The data was analyzed and normalized by using GeneSpring GX, version 11 (Agilent Technologies, Santa Clara, CA, USA).
Animal studies assessing the anti-cancer activities of CPI-613
CD1-Nu/Nu female mice, 28 days old obtained from Charles River Laboratories, were used. Mice were housed 5 to a cage in a micro-isolator room in the Department of Animal Laboratory Research of New York State University at Stony Brook. Light-dark cycles were 12 h each daily, with light from 7 a.m. to 7 p.m. Food (Purina Rodent Chow) and water (distilled sterile-filtered water, pH 7) were provided ad libitum. An acclimation period of 7 days was allowed between the arrival of the animals at the study site and tumor inoculation, before the animals were used in experimentation. Mice were then inoculated subcutaneously in the right flank with 2×106 human pancreatic BxPC-3 tumor cells. The tumor cells were suspended in 0.1 mL of Dulbeco’s Phosphate Buffered Salt (PBS) solution, and inoculation into the mice was performed using a 1 cc syringe with a 27 5/8 gauge needle. The dimensions (length and width) of the tumor mass in the right flank of mice were measured using Vernier calipers up to three times weekly during the study and the tumor volume was calculated using the prolate ellipsoid formula: (length × width2)/2. Test agents were administered via intraperitoneal injection, using a 3 cc syringe with a 25 5/8 gauge needle, once per week on Tuesday for four consecutive weeks beginning when the tumor mass was approximately 90-100 mm3. Body weight was determined immediately prior to administration of test agents. The mice were monitored daily for physical conditions and mortality. Per Institutional Animal Care and Use Commit (IACUC) rules, mice were euthanized if the tumor size exceed 2 cm in diameter; display symptoms of pain or distress; evident by sudden rapid weight loss (>15% of original weight); lethargic behavior; difficulty in moving; vocalization; changes in respiratory rate, overall appearance and activity; signs of erythema, ulceration, infection, necrosis of the tumor; unable to reach food and water; and symptoms of pain or distress. Survival assessment was based on mice that did not die, or were not euthanized per IACUC rules.
Early clinical studies
The anti-cancer activities, according to OS, of various doses of CPI-613 alone and in combination with Gemcitabine (1,000 mg/m2), was assessed in patients with Stage IV pancreatic cancer. In the clinical trial in which CPI-613 alone were assessed (Study# CL-CPI-613-002, clinicaltrials.gov identifier NCT007414403), various dose of CPI-613 (range, 280-1,300 mg/m2) was given 2× weekly for 3 weeks, followed by a week of rest. In the clinical trial in which “CPI-613 + Gemcitabine” combination was assessed (Study# CL-CPI-613-004, clinicaltrials.gov identifier NCT00907166), various doses of CPI-613 (ranging 70-320 mg/m2) was given 2× weekly, whereas Gemcitabine (1,000 mg/m2) was given 1× weekly, for 3 weeks followed by a week of rest. The studies adhered to the Declaration of Helsinki and the International Conference on Harmonisation (ICH) Harmonized Tripartite Guidelines for Good Clinical Practice. All patients were recruited from the clinical practice in the US and Canada between August 2009 and December 2010. Approval was obtained from Independent Ethics Committee (IEC) or local Institutional Review Boards (IRB) at each study site. Written informed consent was obtained from each subject.
Calculation and statistical analysis
For the microarray studies, genes with an associated P value <0.05 were considered statistically significant and termed differentially expressed genes. A 1.5-fold was used as the cut-off. For the animal studies, tumor volume and body weight were tabulated. The results, expressed as mean ± standard error of the mean, were graphically presented (SigmaPlot 11.0, Systat Software, Inc., San Jose, CA, USA). The animal and clinical survival data was tabulated and the animal data were graphically presented (SigmaPlot 11.0). For the tumor volume data of the animal studies, differences among various treatment groups at various times were analyzed using Analysis of Various (ANOVA) with repeated measures. For the survival data, they were summarized using the Kaplan-Meier method and differences among treatment groups were compared using the LogRank test. Differences were considered to be significant if P value was <0.05. For the early clinical studies, survival data were not statistically analyzed because of the small sample size.
Results
Cell cycle gene profiles affected by CPI-613 according to microarray technique
Table 1 lists the cell-cycle regulating genes with fold changes ≥1.5 units induced by CPI-613 or sham treatment. Microarray profiling revealed that CPI-613 down-regulated the expression of Cyclin D3, E1, E2, F, A2, B1 and CDK2 genes, when compared to sham treatment. The expression of these genes in non-transformed NIH-3T3 mouse fibroblast cells (the negative control in these experiments) was not affected.
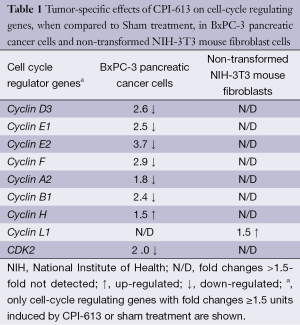
Full table
Animal efficacy studies
Figure 1 shows the tumor volumes of mice with pancreatic tumor xenografts treated with CPI-613, Gemcitabine or vehicle. Both CPI-613 and Gemcitabine suppressed the growth of pancreatic tumor xenografts, when compared to control. Tumor growth inhibition of both agents occurred not only during treatment, but also for at least 4 weeks after treatment. However, the anti-tumor effects tended to be greater for CPI-613 than Gemcitabine.
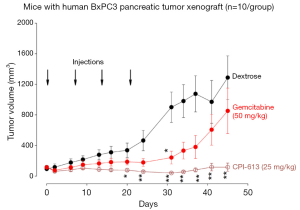
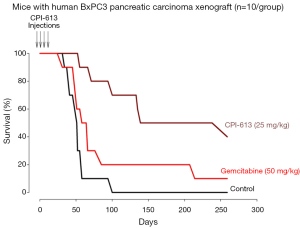
Figure 2 shows the survival curves of mice with pancreatic tumor xenografts treated with CPI-613, Gemcitabine or vehicle. The median survival (95% confidence interval) were 50.0 (range, 41.5-58.5) days for the D5W group, 138.0 (range, 55.7-331.7) days for the CPI-613 group; and 65.0 (range, 50.8-79.2) days for the Gemcitabine group. The survival of the CPI-613 group was significantly longer than the D5W group (P=0.0170) and tended to be longer than the Gemcitabine group (P=0.0500). The survival of the Gemcitabine group was also significantly longer than the D5W group (P=0.0253). These results indicate that both CPI-613 and Gemcitabine prolonged survival of the tumor bearing mice, when compared to control.
Early clinical studies
In the clinical trial in which CPI-613 as monotherapy was assessed, there were four patients with advanced pancreatic cancer (Table 2). All four patients were elderly Caucasian males who were at least 62 years of age, had received 2-3 lines of chemotherapies prior to participating in the trial, and CPI-613 was the last chemotherapy for their cancer. The doses of CPI-613 used in these pancreatic cancer patients were between 280 to 1,300 mg/m2.
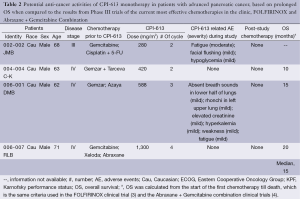
Full table
No CPI-613 related adverse events were observed in two of these four patients, whereas mild and transient CPI-613 related adverse events were observed in the other two patients. The CPI-613 related adverse events included fatigue/weakness, flushing, hypoglycemia, hyperkalemia, absence of breath sounds in the lower half of lungs, and rhonchi in left upper lung. The last two adverse events were related to the lung, which were likely due to the presence of large tumor lesions in the lung of the patient. Due to their mild and transient nature, none of the adverse events were considered dose limiting.
OS of these patients was calculated from the start of the first chemotherapy till death, which is the same criteria used in the Phase III FOLFIRINOX trial (4) and the Phase III trial of Abraxane + Gemcitabine combination (3). OS of the first of these study subjects (i.e., Subject 002-002/JMB) was not available. For the remaining 3 subjects, the median OS was 15 months which is longer than the median OS of the metastatic pancreatic cancer patients in the clinical trials of Abraxane + Gemcitabine combination and FOLFININOX, which was 8.5 and 12.2 months, respectively. The longer median OS associated with CPI-613 treatment in this clinical trial, than those of FOLFIRINOX and Abraxane + Gemcitabine treatment, was in spite of the fact that FOLFIRINOX and Abraxane + Gemcitabine were used as first-line therapies in their Phase III trials whereas CPI-613 was used as the last-line therapy in the current trial. Also, the OS values of the 3 OS-evaluable pancreatic cancer patients in this trial were linearly dose-related [coefficient correlation (or r value) being 0.942].
In the clinical trial in which patients were treated with CPI-613 + Gemcitabine combination, there were six patients with metastatic pancreatic cancer (Table 3). These patients included both sexes and were of different ethnic backgrounds. All but the first patients were elderly. Three of these six patients underwent 1-3 lines of chemotherapies, whereas the other three patients did not undergo any chemotherapies prior to participating in the trial. CPI-613 + Gemcitabine combination was the last therapy for their cancer in all six patients. The dose of CPI-613 used in these six patients was 70-320 mg/m2, and the dose of Gemcitabine was 1,000 mg/m2.
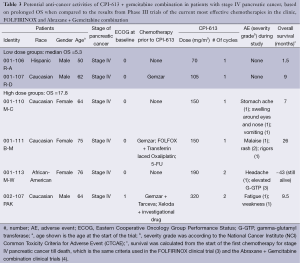
Full table
No CPI-613-related adverse events were observed in the two patients in the low dose group (CPI-613 dose being 70-105 mg/m2). The four patients in the high dose group (CPI-613 dose being 150-320 mg/m2) experienced the following CPI-613 related adverse events: stomach ache, swelling around the eyes and nose, vomiting, malaise, rash, rigors, headache, elevated G-GTP, and fatigue/weakness. Rash and elevated G-GTP were of Grades 2 and 3 severity respectively, whereas all other adverse events were of Grade 1 severity, per National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Event (CTCAE). All adverse events were reversible.
OS of these six pancreatic cancer patients was calculated from the start of the first chemotherapy till death, which is the same criteria used in the FOLFIRINOX (3) and the Abraxane + Gemcitabine combination (4). Median OS of the first two patients treated with low doses of CPI-613 (70 and 105 mg/m2) is 5.3 months, which is comparable to the median OS of 5.6 months associated with gemcitabine (27). Therefore, CPI-613 did not make any contribution beyond Gemcitabine. The median OS of the 4 patients treated with higher doses of CPI-613 (150-320 mg/m2) is 17.8 months, which is better than the median OS of 12.2 and 8.5 months in patients treated with FOLFIRINOX and Abraxane+Gemcitabine combination, respectively (3,4). As a matter of fact, Patient 001-113/M-W, who received CPI-613 (190 mg/m2) + Gemcitabine combination as the only chemotherapy for her cancer, exhibits an OS of ~43 months and this patient is still alive as of February 2014.
Discussion
The current manuscript describes results from translational assessment of mitochondrial dysfunctional of pancreatic cancer, from in vitro gene microarray and animal efficacy studies to early clinical studies, via the tumor-specific anti-mitochondrial agent CPI-613.
Rationale for choosing BxPC-3 pancreatic cancer cell line in the current translational investigation
Human BxPC-3 tumor cell line was chosen for the in vitro and animal efficacy studies in the current translational investigation. This was because BxPC-3 cells are pancreatic adenocarcinoma (28,29), and pancreatic adenocarcinoma represents ~90% cases of pancreatic cancer presented in the clinic. Furthermore, these tumor cells were isolated from the head or neck of the pancreas (28,29), which represent the majority of the pancreatic adenocarcinoma. Choosing a pancreatic cancer cell line that represents the majority of cases in the clinic and the majority of pancreatic adenocarcinoma should provide better translational investigation from in vitro and animal studies to clinical studies.
Microarray studies
Microarray studies indicated that the expression of Cyclin D3, E1, E2, F, A2, B1 and CDK2 genes of BxPC-3 pancreatic cancer cells was down-regulated by CPI-613 but not sham treatment. The down-regulation of these genes by CPI-613 also did not occur with the non-transformed NIH-3T3 mouse fibroblast cells (the negative control). Cyclin D3 and Cyclin E genes are known to regulate the conversion of cells from G1 to S phase, Cyclin A gene from S to G2 phase, and Cyclin B genes from G2 to M phases, during cell cycles. Further, the CDK2 gene is necessary for progression of cells from G1 to S phase, and from S to G2 phase, during cell cycles. Due to down-regulation of these cell-cycle regulating genes at multiple points, cell-cycle progression of BxPC-3 pancreatic cancer cells were probably halted thus leading to the death of these cancer cells. In short, genetic alterations detected by microarray technique were translational to the anti-cancer activities observed in animal efficacy studies and early clinical studies.
Animal efficacy studies
The animal efficacy studies showed prolonged inhibition of tumor growth and prolongation of survival by only four weekly administrations of CPI-613 in mice with pancreatic carcinoma xenografts. These results are consistent with the microarray data described above and the clinical data described below. The anti-cancer activities were more effective with CPI-613 (a tumor-specific anti-mitochondrial agent) than Gemcitabine (a pyrimidine anti-metabolite that inhibit DNA synthesis), since the degree of tumor growth inhibition was ~2×, and prolongation of survival was ~4×, greater with CPI-613 treatment than Gemcitabine treatment.
Early clinical studies
After treatment with CPI-613 as monotherapy, the median OS of three patients with Stage IV advanced pancreatic cancer had prolonged and was 15 months. After treatment with high doses of CPI-613 in combination with Gemcitabine, the median OS of four patients with Stage IV advanced pancreatic cancer was 17.8 months. These median OS values tend to be longer than the reported median OS of patients treated with the most effective chemotherapies currently available in the clinic for pancreatic cancer, Abraxane + Gemcitabine combination (4) and FOLFIRINOX (3), which was 8.5-12.2 months. The prolonged OS from CPI-613 treated patients was superior to that of the current most effective chemotherapies in the clinic suggesting anti-cancer activities of CPI-613. In spite of the small number of patients from early clinical study, these results are consistent with the gene microarray and animal efficacy studies.
There are a number of reasons that OS (instead of response criteria based on imaging scans or progression-free-survival based on imaging scan-derived response criteria) is used to compare the anti-cancer activities of CPI-613 and CPI-613 + Gemcitabine combination from the current studies to that of the current most effective chemotherapies Abraxane + Gemcitabine combination and FOLFIRINOX. First, OS is the gold standard to reflect anti-cancer activities of anti-cancer drugs, superior to any surrogate endpoints. Second, CPI-613 has been shown to induce pseudo-progression of cancer disease when response criteria based on imaging scans are used to assess treatment response due to induction of inflammation and necrosis at tumor sites by CPI-613 causing the tumor lesions to be enlarged and persistent (23), thus making imaging-based response criteria unreliable to indicate anti-cancer activities.
Dose-limiting toxicities of CPI-613 were not observed in patients described in this manuscript, due to the mild and transient nature of their adverse events. DLT of CPI-613 were observed in a different clinical trial in patients with hematologic malignancies in which the safety of CPI-613 at doses as high as 3,780 mg/m2 was assessed (unpublished observation). In this trial, DLT was found to be renal failure and occurred in 2 of 3 patients treated at 3,780 mg/m2. Renal failure was resolved except in one of these two patients who opted out of treatment and chose hospice care.
Summary
In summary, The dysfunctional mitochondria of pancreatic cancer cells was translationable from in vitro gene alteration and animal tumor model studies to patients with advanced Stage IV pancreatic cancer, as reflected by the anti-cancer activities of the tumor-specific anti-mitochondrial agent, CPI-613, in these studies.
Acknowledgements
The data described in this manuscript has not been presented or published, except as a poster presentation. The data described in this manuscript was not supported by funds from any outside source.
Disclosure: All authors are employees of Cornerstone Pharmaceuticals, Inc. who develops the test agent, CPI-613, used in this study. The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008;8:82. [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N Engl J Med 2011;364:1817-25. [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [PubMed]
- Lee W, Lockhart AC, Kim RB, et al. Cancer pharmacogenomics: powerful tools in cancer chemotherapy and drug development. Oncologist 2005;10:104-11. [PubMed]
- Kleeff J, Michalski C, Friess H, et al. Pancreatic cancer: from bench to 5-year survival. Pancreas 2006;33:111-8. [PubMed]
- Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321:1801-6. [PubMed]
- Caldas C, Hahn SA, da Costa LT, et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet 1994;8:27-32. [PubMed]
- Iacobuzio-Donahue CA, Song J, Parmiagiani G, et al. Missense mutations of MADH4: characterization of the mutational hot spot and functional consequences in human tumors. Clin Cancer Res 2004;10:1597-604. [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [PubMed]
- Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res 2009;15:4674-9. [PubMed]
- Dan S, Tsunoda T, Kitahara O, et al. An integrated database of chemosensitivity to 55 anticancer drugs and gene expression profiles of 39 human cancer cell lines. Cancer Res 2002;62:1139-47. [PubMed]
- Zembutsu H, Ohnishi Y, Tsunoda T, et al. Genome-wide cDNA microarray screening to correlate gene expression profiles with sensitivity of 85 human cancer xenografts to anticancer drugs. Cancer Res 2002;62:518-27. [PubMed]
- Staunton JE, Slonim DK, Coller HA, et al. Chemosensitivity prediction by transcriptional profiling. Proc Natl Acad Sci U S A 2001;98:10787-92. [PubMed]
- Cui Y, Brosnan JA, Blackford AL, et al. Genetically defined subsets of human pancreatic cancer show unique in vitro chemosensitivity. Clin Cancer Res 2012;18:6519-30. [PubMed]
- Bingham PM, Zachar Z. The Pyruvate Dehydrogenase Complex in Cancer: Implications for the Transformed State and Cancer Chemotherapy. In: Dehydrogenases. Edited by Canuto RA. New York: Intech, 2012. Chapter 3.
- Zachar Z, Marecek J, Maturo C, et al. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J Mol Med (Berl) 2011;89:1137-48. [PubMed]
- Stuart SD, Schauble A, Gupta S, et al. A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer Metab 2014;2:4. [PubMed]
- Pardee TS, Stadelman K, Isom S, et al. A phase I study of the mitochondrial metabolism inhibitor CPI-613 in combination with high-dose ara-C (HDAC) and mitoxantrone for relapsed or refractory acute myeloid leukemia (AML). [abstract]. J Clin Oncol 2014;32:abstr 7028.
- Pardee TS, Levitan DA, Hurd DD. Altered mitochondrial metabolism as a target in acute myeloid leukemia [abstract]. J Clin Oncol 2011;29:abstr 6590.
- Pardee TS, DeFord-Watts LM, Peronto E, et al. Evaluation of the first-in-class antimitochondrial metabolism agent CPI-613 in hematologic malignancies [abstract]. J Clin Oncol 2012;30:abstr 6524.
- Lee K, Khaira D, Rodriguez R, et al. Long-Term Stable Disease of Stage IV Pancreatic Neuroendocrine Tumors and Without Significant Adverse Effect by CPI-613, an Investigational Novel Anti-Cancer Agent. Case Study and Case Report 2011;1:137-45.
- Lee K, Maturo C, Luddy J, et al. Pseudo-progression of metastatic pancreatic cancer assessed by imaging studies - a case report. Case Study and Case Report 2012;2:95-101.
- Senzer N, Bedell C, Maturo C, et al. CPI-613, an investigational novel anti-cancer agent, provides long-term stable disease without significant adverse effects in a patient with stage IV relapsed hepatocellular carcinoma. Case Study Case Rep 2012;2:38-45.
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029-33. [PubMed]
- Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol 1927;8:519-30. [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [PubMed]
- Chen WH, Horoszewicz JS, Leong SS, et al. Human pancreatic adenocarcinoma: in vitro and in vivo morphology of a new tumor line established from ascites. In Vitro 1982;18:24-34. [PubMed]
- Tan MH, Nowak NJ, Loor R, et al. Characterization of a new primary human pancreatic tumor line. Cancer Invest 1986;4:15-23. [PubMed]


