
The modern approach to esophageal palliative and emergency surgery
Introduction
Historically, the management of esophageal palliative and emergency conditions was primarily achieved via invasive surgery and was associated with high mortality and morbidity. With advances in endoscopic and systemic therapies, the management of these complex conditions has changed, and the outcome of these patients has significantly improved. As a result of these advances, the role of thoracic surgeons in treating these diseases has evolved. Thoracic surgeons have recognized the importance of advanced endoscopic skills and have developed expertise in the techniques and skill sets required for advanced endoluminal procedures. Thoracic surgeons currently have multiple options and strategies to guide treatment in these cases.
In this review, a modern approach to esophageal palliative and emergency surgery is discussed. Key principles of endoluminal procedures from the literature, recent guidelines and innovations on the subject are presented.
Palliative esophageal cancer surgery in the modern era
Presently, more than half of patients with esophageal cancer are initially diagnosed at an inoperable stage (1).
A cornerstone of palliative care is to integrate it early in order to have an impact on functional, physical and psychosocial status (2). Palliative care aims at improving quality of life (QOL) and symptoms with local and systemic therapies (3,4). In esophageal cancer, most of the palliative treatment options aim at reducing dysphagia which is the most common symptom in inoperable patients. Dysphagia is mainly caused by obstruction of the lumen of the esophagus or gastroesophageal junction by tumor. Dysphagia has a significant adverse effect on QOL and prognosis of patients (5,6). For many inoperable patients, dysphagia is related to weight loss, regurgitation, aspiration pneumonia and, it can even lead to withdrawal from social situations (5,6).
To guide the selection of an individualized palliative approach, physicians, including thoracic surgeons, must take into consideration many factors including prognosis, performance status and comorbidities of patients. For dysphagia more specifically, the Cochrane review updated in 2014 and the European Society of Gastrointestinal Endoscopy (ESGE) Clinical Endoscopy recommend self-expanding metal stent (SEMS) as a safe, effective and expedient modality for palliating dysphagia compared to other treatment options (Table 1) (7,8). For predicted life expectancy of 3 months or more, guidelines state that high-dose intraluminal brachytherapy (ILBT) is a suitable alternative for dysphagia improvement (7,8), however, in our opinion and in our practice we believe that brachytherapy as palliation of dysphagia should be reserved for very selected cases as stenting is quicker, requires only one hospital visit, significantly cheaper and associated with immediate dysphagia relief.
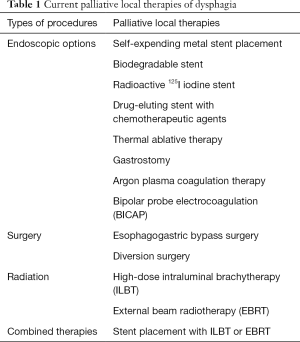
Full table
Surgery as a palliative strategy for esophageal cancer
Currently, surgery as a palliative strategy should almost never be considered because of the high mortality and morbidity associated with such a procedure and the endoscopic options which are available. In a series of patients operated with intrathoracic esophageal carcinoma complicated by fistula, the operative morbidity was 40.0% (14 of 35 patients) and postoperative mortality was 14.3% (5 of 35 patients) (9).
With recent studies on biological therapies and comprehensive molecular analysis of esophageal cancer by the Cancer Genome Atlas Research Network in 2017 (10), a shift toward restaging and potential curative intent is occurring in current clinical practice. In case series, promising results are presented of potentially curative surgery after downstaging of initially inoperable esophageal cancer cases, i.e., invasion of surrounding structures or oligometastatic disease cases (11). A systemic review from nonrandomized studies from Japan observed, in carefully selected esophageal cancer patients (mainly squamous cell carcinoma) with invasion of surrounding structures, a 1-, 3-, 5-year overall survival rates of 24–100%, 5–50% and 0–51%, respectively, after downstaging with definitive chemoradiotherapy and surgery (12,13). Prognostic determinants were the pathological response to multimodal therapies and an R0 resection.
In salvage surgery cases, a prior multidisciplinary discussion and restaging are required following induction before proceeding to surgery. A cautiously planned surgery, at experienced centers, implies a proper conduit and alternatives, the use of omental tissue to cover and protect anastomoses, an anastomosis preferably outside of the radiation field and, the possibility of a staging resection and reconstruction (14).
Endoluminal procedures as a palliative strategy for esophageal cancer
Esophageal stent placement versus other endoscopic modalities
Esophageal stent placement (Figure 1) is the most widely used intervention for rapidly relieving dysphagia in inoperable esophageal cancer patients (7,8). By maintaining oral intake with no or a short hospital stay, esophageal stenting allows palliative patients to improve their QOL (15).
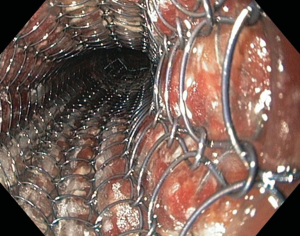
The Cochrane review on dysphagia from esophageal cancer (3,684 patients from 53 studies) recommends as an initial approach SEMS over others endoscopic and surgical modalities, i.e., plastic tube placement, thermal and chemical ablative therapies (7). A similar recommendation was made by the ESGE Clinical Guidelines group. Other modalities are associated with increased requirements for re-interventions and complications (Table 2) (7,8).

Full table
In the literature, high-level evidence-based studies comparing characteristics of different types of esophageal stents are lacking. Esophageal stent selection in the management of dysphagia requires an individualized approach. Tumor characteristics (i.e., position, length and degree of obstruction) and stent designs (i.e., materials, axial rigidity and radial force) are factors to consider during esophageal stent selection (4,18). For example, different radial forces at the gastroesophageal junction are observed depending on type of esophageal stent selected (19). Radial force can explain retrosternal pain, reflux and intolerance of certain patients to a newly inserted stent (19). For stent design, manometry measurements from endoflip measurements demonstrate that SEMS in patients with adenocarcinoma at the gastroesophageal junction do not fully expand to the esophageal wall (19). Therefore, possible lateral impaction can occur (19). With future innovations which will hopefully allow for improvement in geometrical and mechanical properties of esophageal stents, symptoms related to stent insertion can hopefully be improved.
Esophageal stent placement versus ILBT
A recommended initial alternative to esophageal stent placement, in patients with dysphagia and good predicted life expectancy, is ILBT (7,8). This recommendation is mainly based on the Dutch SIREC study group published in 2004. In this study, inoperable patients with esophageal cancer were randomly assigned to stent placement or single-dose 12-Gy brachytherapy to investigate the relief of dysphagia (16). The authors demonstrated that dysphagia improved more rapidly after stent placement (1 to 2 days), but ILBT had longer-lasting relief of dysphagia. At 30 days after treatment, improvement of dysphagia was observed in 76% (70 of 92 patients) of patients in the stent placement group and 73% (64 of 88 patients) in ILBT group (P=0.61). For QOL, the emotional, cognitive, and social functioning were significantly better, during the follow-up, in ILBT group (P<0.05). In the stent placement group, complications were significantly more frequent compared to ILBT (33% vs. 21%, P=0.02) (Table 2). The stent placement group had more bleeding than the ILBT group with 13% (vs. 5% in ILBT patients). Unfortunately, not all hemorrhage cases, in this study, underwent an endoscopy to identify the cause of bleeding. Median survival was 145 days (95% CI: 103–187) in the stent placement group and 155 days (95% CI: 127–183) in the ILBT group (P=0.23).
Although the total medical cost generated by esophageal stent placement or a single 12-Gy ILBT were similar in the Dutch SIREC study group, the number of sessions of ILBT have a direct impact on the medical cost. Health economic evaluation of ILBT with 3 sessions of 7 Gy significantly increased the median medical cost in the ILBT group (20).
The accessibility of ILBT is a concern with limited availability and need for local expertise (8). The nationwide Netherlands Cancer Registry confirmed that only 6% of patients with inoperable esophageal cancer had ILBT, between 2001 and 2010, as initial palliative approach. External beam radiotherapy (EBRT), in this registry, was more frequently employed for relief of dysphagia (3). Although fractionated EBRT is frequently used as an alternative to ILBT because of its availability, there is no high-level quality evidence-based (multiple randomized studies) comparing EBRT and stent placement for the relief of dysphagia in inoperable esophageal cancer patients.
In summary, SEMS, in the modern era, is the initial local procedure of choice recommended for the palliative care of dysphagia in inoperable esophageal cancer patients. Knowledge regarding the properties, limitations and complications of esophageal stenting are required by thoracic surgeons in current practice.
A shift toward combined therapies
Currently, patients with inoperable esophageal cancer have access to oncological and biological therapies that are improving their prognosis. In the UK, it was demonstrated using the UK Registry of Esophageal Stenting (ROST), that 60% of patients surviving more than 6 months required further procedures for dysphagia (21). Combined therapies were also reported in the Dutch SIREC study group with management of tumor overgrowth cases after initial treatment (11 of 16 cases had a second stent placement; 19 of 26 cases of ILBT had a stent placement) (16). This emphasizes the role for a multidisciplinary team approach in the management of palliative care of esophageal cancer patients and the importance of combining therapies.
The combination of esophageal stent placement with other therapies has an impact on palliative care. The combination strategy includes esophageal stent placement with ILBT or EBRT. In a meta-analysis performed on the subject (8 randomized studies enrolling 732 patients), stent combination therapy compared to stent alone was associated with favorable overall survival, longer-lasting relief of dysphagia and QOL improvement (22). Less complications occur in the stent combination therapy group, including stent migration, aspiration pneumonia and tumor overgrowth. However, the ESGE Clinical Guidelines report contradictory data on complications using combined therapies and do not recommend combination therapy (8). Particularly for EBRT and stent placement, major complications, i.e., tracheoesophageal fistula and hemorrhage, were reported in retrospective studies (23). Additional data are expected from the ROCS study which is currently ongoing. ROCS is a phase 3 study that assesses the relief of dysphagia after the combination of EBRT and stent placement. Patient recruitment finished in 2018 (24).
Tracheoesophageal and bronchoesophageal fistulas (TEF)
TEF (Figure 2) are reported in 5% to 15% of esophageal cancer cases. TEF are associated with aspiration pneumonia and poor nutritional intake (8). The mean survival, in the literature, ranges between 1 and 6 weeks (8,25-28). The most widely used approach is the endoscopic approach with endoluminal stenting with the goal of excluding the fistula. Other modalities, in contemporary studies, are gastrostomy (with or without tracheostomy) and bypass surgery. As mentioned earlier, esophagectomy in these frail patients is associated with very high mortality and morbidity rates and is not recommended (9).
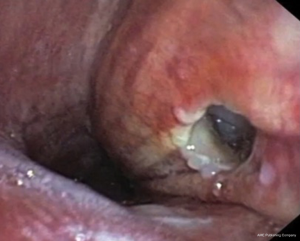
Stenting to exclude a TEF allows successful and rapid sealing in more than 75% of cases (8). A double stenting approach (airway and esophageal stents) is considered when a fistula is not fully covered by a single stent (Figure 3). It permits concomitant relief of both airway and esophageal symptoms. The safety and efficacity of double stenting to relieve symptoms related to fistulas have been reported in cohort studies (26-28). In these studies, the performance status of patients with a TEF usually improves after stenting.
We typically attempt esophageal stenting alone in TEF and reserve double stenting for patients who either do not have complete fistula coverage and exclusion following esophageal stenting or patients with bulky peri-tracheal/bronchial disease with airway compromise and stridor who require concomitant airway palliation in addition to TEF exclusion. When attempting to exclude a TEF with endoluminal esophageal stenting, it is vital to perform a bronchoscopy before and after esophageal stent placement during the same procedure. Esophageal stenting can create luminal airway compromise by pushing on the tumor and require either immediate esophageal stent removal or concomitant airway stenting. The same principal applies for esophageal stenting for any tumor in the proximal or mid esophagus where the esophageal tumor location is near the trachea, carina or mainstem bronchi.
Innovations in esophageal stenting for palliation
Presented here are summarized data on innovations in esophageal stenting. These innovations will require further evaluation and validation studies.
Radioactive stent
Radioactive iodine SEMS have been created to combine the rapid relief of dysphagia of SEMS with the prolong effect of radiation on dysphagia. In a recently published meta-analysis, irradiation stents compared to conventional stents have an increase median overall survival of 2.734 months (95% CI: 1.71–3.775, P<0.005) (17). Patients with an irradiation stent reportedly have better relief of dysphagia at 3 and 6 months. No significant difference is reported for complications, including bleeding, perforation or fistula formation.
Drug-eluding stent
Drug-eluding stents containing chemotherapeutic agents are still in an experimental phase. A recent phase 1 study, from China, on rabbit esophagus demonstrated that paclitaxel from a drug-eluting stents can be magnetocalorically released and can effectively penetrate the esophagus wall (29). Further studies on this future perspective are required.
Biodegradable stent
Esophageal biodegradable stents were initially developed for benign esophageal strictures. The dissolving properties within 3 months of insertion sparked interest as potentially useful in the palliative care of esophageal cancer patients due to the possibility of combining them with ILBT. Despite promising results from the BEST study on benign strictures, the safety prospective study performed by Hirdes et al. on inoperable esophageal cancer patients was ended prematurely because the safety threshold of major complications (i.e., severe retrosternal pain, hematemesis and recurrent dysphagia) was reached (30,31). Therefore, the combined treatment of biodegradable stent and ILBT for relief of dysphagia in esophageal cancer patients is currently not recommended. Since 2012, studies on biodegradable stents have mainly been on their experimental use as a bridge to surgery during neoadjuvant treatment in operable esophageal cancer patients (32).
Emergency surgery in the modern era
Acute intrathoracic perforation of the esophagus
The management of esophageal perforations has rapidly evolved since the initial description of an endoluminal procedure for iatrogenic intrathoracic esophageal perforation in 2007 by Freeman (33). The most common etiology of esophageal perforations is iatrogenic causes being responsible of more than 60% of perforations (34,35). The remaining benign esophageal perforations are caused by Boerhaave syndrome (15% to 30%), trauma and foreign body ingestion (34).
Traditional operative surgical procedures for esophageal perforations are associated with morbidity rates as high as 40% at experienced centers (34,36). With a high index of suspicion, multidisciplinary team expertise, antibiotics and hybrid treatment strategies, the outcomes of this complex condition have significantly improved in recent years (34,35,37). The evolution in management of acute esophageal perforations was described by Kuppusamy et al. in 2011. Non-operative treatment went from 0% between 1989 and 1992 to 75% between 2005 and 2009 (P<0.001) (37). These changes in management of esophageal perforations are associated with decreased complications rates (50% to 33%, P=0.94) and reduced length of stay (median length of stay in days 18.5 to 8.5 days, P=0.094).
Hybrid treatment strategies denote the combination of minimally invasive interventions, i.e., thoracoscopy or prompt and effective pleural/mediastinal drainage procedures for source control and endoluminal procedures to seal the esophageal perforation. Endoluminal procedures can be performed under deep sedation or general endotracheal anesthesia. Advantages of hybrid treatment strategies are early post-intervention enteral nutrition (oral intake or enteral feeding tube) and mobilization. In our center, hybrid treatment strategies include percutaneous endoscopic gastrostomy (PEG) or percutaneous endoscopic gastro-jejunostomy (PEG-J) for gastric drainage and early enteral nutrition.
As in any treatment strategy, hybrid treatment approaches have limitations. Experts recommend an operative approach for patients with long segment transmural esophageal injury (>6 cm) and indication for immediate thoracotomy for an associated injury (36).
In our experience, severe hemodynamic instability is an ideal situation for temporization measures with an endoscopic or hybrid approach. This allows for hemodynamic stabilization and rehydration of the patient, invasive monitoring, correction of coagulopathy, re-warming and allowing time for appropriate imaging. Rushing into a major operation, which often leads to worsening hemodynamic status and hypothermia, is associated with high mortality and morbidity, and often, with esophageal defunctionalization, which is a disease in itself. Intensive care unit admission, central and peripheral access and monitoring, catecholamine and fluid administration, active re-warming, bedside endoluminal stenting, pleural and/or mediastinal percutaneous drainage, intravenous antibiotics and proton pump inhibitors lead to significant stabilization and limit mediastinal and pleural soilage. Once stabilized, thoracic and abdominal computed tomography can be performed to decide if further drainage and/or debridement procedures (i.e., percutaneous, thoracoscopic, laparoscopic or via open surgical approaches) are required.
Endoluminal procedures as treatment of acute intrathoracic esophageal perforation
Esophageal stent placement
In intrathoracic esophageal perforation cases, esophageal stent placement is the most widely used and recommended endoluminal procedure (8,35,36). In current practice, stenting is also utilized for anastomotic leak, persistent perforation after open repair and for carefully selected perforation cases in the setting of esophageal malignancy (36).
Clinical success of stent placement to seal perforations can be achieved in more than 85% of patients (Table 3) (38). Esophageal stenting was shown to improve outcomes with the lowest associated mortality rate in a meta-analysis comparing stent placement with primary repair or T-tube repair (7.3% vs. 13.8% vs. 20.0%, respectively with a pooled mortality rate of 13.8%) (39).

Full table
Major complications related to esophageal stent placement for iatrogenic perforations are rare (Table 3) (35). The most common complication is stent migration with an incidence approximately of 20% (35). Other complications reported include tissue overgrowth, erosion or ulceration, bleeding, aspiration pneumonia, perforation, fistula formation and reflux (35).
An important factor to consider is length of treatment. Unfortunately, the optimal time to remove an esophageal stent after an acute perforation is unknown. Historically, the removal of stents occurs between 6 to 8 weeks (38). Although, data from a prospectively collected database, from a single institution, reported a significant decrease of complications in patients with an acute esophageal perforation whose stent was removed in less than 28 days after placement compare with removal time more than 28 days (reduction by 39%, OR 0.61, 95% CI: 0.54–0.78, P<0.01) (40). We currently remove esophageal stents at 2–4 weeks and if a fistula is still present, a new stent is re-inserted at the same setting. Prolonged indwelling esophageal stents can lead to erosion, pressure ischemia/necrosis and fistulization to neighboring organs.
Endoscopic clips
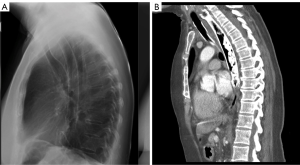
First reported in 2007 on patients with gastric or colonic bleeding or lesions, over-the-scope clips (OTSC) (Ovesco Endoscopy GmbH, Tübingen, Germany) have gain popularity over the last decade (41). With its bear claw appearance, the OTSC allows closure of transmural defect in acute esophageal perforation cases by bunching up nearby tissue to allow esophageal healing without stent placement (Figure 4).
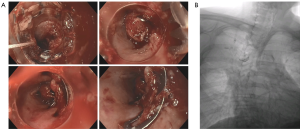
OTSC have a clinical success (i.e., recovery from perforation) ranging from 56% to 100% (Table 3) in nonrandomized studies (35). The success rate depends on types of perforation (90% for acute perforations; 68% for postoperative perforations; 59% for chronic leaks and fistulas, P<0.001) (42). The different success rates observed may be associated with the quality of mucosal edges, i.e., inflammation and flexibility of tissue and the size of the hole. Compared to through-the-scope clips, OTSC have a greater compression force (42) and lesion size closure is approximately 8mm (35).
Complications described with OTSC are malfunction in clip deployment, contralateral esophageal ulceration/laceration and tongue laceration (35,42). There is one report of perforation secondary to OTSC placement technique in literature (35,42).
Endoluminal vacuum (E-Vac) therapy (EVT)
EVT is a negative pressure system with continuous suction (usually between −125 and −175 mmHg) applied to an extra-esophageal cavity by mounting a porous wound sponge (polyurethane foam) attached to a nasogastric tube (35). The sponge is positioned endoscopically in the esophageal lumen or abscess cavity. The sponge is changed every 3 to 5 days.
Initially described in Germany in 2008 for intrathoracic esophageal anastomotic leaks, it was only reported in an American study for esophageal perforations in 2015 (43). EVT has the advantage of maintaining the ability for regular endoscopic inspection of the luminal defect and repeated cavity debridement, therefore maintaining source control (43,44). Vacuum therapy allows reduction of tissue edema and aids in perfusion and granulation of the esophageal wall (43). The overall success rate of EVT, from nonrandomized studies ranges between 83% and 100% with an average of 90% (Table 3) (35,43,44). Average length of therapy ranged from 12 to 36 days with 4 to 9 sponge changes (35). The main complication feared with EVT is the risk of erosion of vessels with associated severe hemorrhage. Stricture caused by granulation tissue is the most common complication reported (35,43,44).
Studies comparing EVT with esophageal stent placement in esophageal perforation concluded that EVT may be as effective as esophageal stent (43). Some of these studies observed less complications in the EVT group, including stricture (43). Practically, EVT is a good alternative to stenting in patients with large leaks where stents cannot completely seal the lumen, in patients with necrotic looking esophageal perforations, in patients with mediastinal abscesses adjacent to the perforation/leak or patients with sepsis in whom covering up an infected leak is undesirable.
Future perspective in the management of esophageal perforation seems to be the combination of endoluminal therapies tailored to the specific clinical scenario. Endoluminal therapies can be combined with surgical and/or percutaneous drainage/debridement techniques. Effective combination of OTSC and EVT has been reported (43).
Conclusions
Thoracic surgeons benefit from mastering endoluminal therapies and advanced endoscopic techniques. An understanding of these rapidly evolving therapies, i.e., outcomes, limitations and innovations, is required to optimally manage esophageal palliative and emergency conditions.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.107). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Temel JS, Greer JA, El-Jawahri A, et al. Effects of Early Integrated Palliative Care in Patients With Lung and GI Cancer: A Randomized Clinical Trial. J Clin Oncol 2017;35:834-41. [Crossref] [PubMed]
- Opstelten JL, de Wijkerslooth LR, Leenders M, et al. Variation in palliative care of esophageal cancer in clinical practice: factors associated with treatment decisions. Dis Esophagus 2017;30:1-7. [PubMed]
- Siersema PD. New developments in palliative therapy. Best Pract Res Clin Gastroenterol 2006;20:959-78. [Crossref] [PubMed]
- Blazeby JM, Williams MH, Brookes ST, et al. Quality of life measurement in patients with oesophageal cancer. Gut 1995;37:505-8. [Crossref] [PubMed]
- Darling GE. Quality of life in patients with esophageal cancer. Thorac Surg Clin 2013;23:569-75. [Crossref] [PubMed]
- Dai Y, Li C, Xie Y, et al. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev 2014;CD005048 [PubMed]
- Spaander MC, Baron TH, Siersema PD, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016;48:939-48. [Crossref] [PubMed]
- Davydov M, Stilidi I, Bokhyan V, et al. Surgical treatment of esophageal carcinoma complicated by fistulas. Eur J Cardiothorac Surg 2001;20:405-8. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
- Mourikis TP, Benedetti L, Foxall E, et al. Patient-specific cancer genes contribute to recurrently perturbed pathways and establish therapeutic vulnerabilities in esophageal adenocarcinoma. Nat Commun 2019;10:3101. [Crossref] [PubMed]
- Makino T, Doki Y. Treatment of T4 esophageal cancer. Definitive chemo-radiotherapy vs chemo-radiotherapy followed by surgery. Ann Thorac Cardiovasc Surg 2011;17:221-8. [Crossref] [PubMed]
- Makino T, Yamasaki M, Tanaka K, et al. Treatment and clinical outcome of clinical T4 esophageal cancer: A systematic review. Ann Gastroenterol Surg 2019;3:169-80. [Crossref] [PubMed]
- Marks J, Rice DC, Swisher SG. Salvage esophagectomy in the management of recurrent or persistent esophageal carcinoma. Thorac Surg Clin 2013;23:559-67. [Crossref] [PubMed]
- Homs MY, Kuipers EJ, Siersema PD. Palliative therapy. J Surg Oncol 2005;92:246-56. [Crossref] [PubMed]
- Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364:1497-504. [Crossref] [PubMed]
- Chen HL, Shen WQ, Liu K. Radioactive self-expanding stents for palliative management of unresectable esophageal cancer: a systematic review and meta-analysis. Dis Esophagus 2017;30:1-16. [Crossref] [PubMed]
- van der Bogt RD, Vermeulen BD, Reijm AN, et al. Palliation of dysphagia. Best Pract Res Clin Gastroenterol 2018;36-37:97-103. [Crossref] [PubMed]
- Mbah N, Philips P, Voor MJ, et al. Optimal radial force and size for palliation in gastroesophageal adenocarcinoma: a comparative analysis of current stent technology. Surg Endosc 2017;31:5076-82. [Crossref] [PubMed]
- Wenger U, Johnsson E, Bergquist H, et al. Health economic evaluation of stent or endoluminal brachytherapy as a palliative strategy in patients with incurable cancer of the oesophagus or gastro-oesophageal junction: results of a randomized clinical trial. Eur J Gastroenterol Hepatol 2005;17:1369-77. [Crossref] [PubMed]
- Kaltsidis H, Mansoor W, Park JH, et al. Oesophageal stenting: Status quo and future challenges. Br J Radiol 2018;91:20170935 [Crossref] [PubMed]
- Lai A, Lipka S, Kumar A, et al. Role of Esophageal Metal Stents Placement and Combination Therapy in Inoperable Esophageal Carcinoma: A Systematic Review and Meta-analysis. Dig Dis Sci 2018;63:1025-34. [Crossref] [PubMed]
- Nishimura Y, Nagata K, Katano S, et al. Severe complications in advanced esophageal cancer treated with radiotherapy after intubation of esophageal stents: a questionnaire survey of the Japanese Society for Esophageal Diseases. Int J Radiat Oncol Biol Phys 2003;56:1327-32. [Crossref] [PubMed]
- Adamson D, Blazeby J, Nelson A, et al. Palliative radiotherapy in addition to self-expanding metal stent for improving dysphagia and survival in advanced oesophageal cancer (ROCS: Radiotherapy after Oesophageal Cancer Stenting): study protocol for a randomized controlled trial. Trials 2014;15:402. [Crossref] [PubMed]
- Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am 2003;13:271-89. [Crossref] [PubMed]
- Nasir BS, Tahiri M, Kazakov J, et al. Palliation of Concomitant Tracheobronchial and Esophageal Disease Using a Combined Airway and Esophageal Approach. Ann Thorac Surg 2016;102:400-6. [Crossref] [PubMed]
- Roseira J, Mao de Ferro S, Moleiro J, et al. Utility of stent double palliation for esophageal cancer with airway involvement: the extremis of care. Dis Esophagus 2020;33:doz087 [Crossref] [PubMed]
- Włodarczyk JR, Kuzdzal J. Safety and efficacy of airway stenting in patients with malignant oesophago-airway fistula. J Thorac Dis 2018;10:2731-9. [Crossref] [PubMed]
- Jin Z, Wu K, Hou J, et al. A PTX/nitinol stent combination with temperature-responsive phase-change 1-hexadecanol for magnetocaloric drug delivery: Magnetocaloric drug release and esophagus tissue penetration. Biomaterials 2018;153:49-58. [Crossref] [PubMed]
- Repici A, Vleggaar FP, Hassan C, et al. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc 2010;72:927-34. [Crossref] [PubMed]
- Hirdes MM, van Hooft JE, Wijrdeman HK, et al. Combination of biodegradable stent placement and single-dose brachytherapy is associated with an unacceptably high complication rate in the treatment of dysphagia from esophageal cancer. Gastrointest Endosc 2012;76:267-74. [Crossref] [PubMed]
- van den Berg MW, Walter D, de Vries EM, et al. Biodegradable stent placement before neoadjuvant chemoradiotherapy as a bridge to surgery in patients with locally advanced esophageal cancer. Gastrointest Endosc 2014;80:908-13. [Crossref] [PubMed]
- Freeman RK, Van Woerkom JM, Ascioti AJ. Esophageal stent placement for the treatment of iatrogenic intrathoracic esophageal perforation. Ann Thorac Surg 2007;83:2003-7; discussion 2007-8. [Crossref] [PubMed]
- Carrott PW Jr, Low DE. Advances in the management of esophageal perforation. Thorac Surg Clin 2011;21:541-55. [Crossref] [PubMed]
- Watkins JR, Farivar AS. Endoluminal Therapies for Esophageal Perforations and Leaks. Thorac Surg Clin 2018;28:541-54. [Crossref] [PubMed]
- Herrera A, Freeman RK. The Evolution and Current Utility of Esophageal Stent Placement for the Treatment of Acute Esophageal Perforation. Thorac Surg Clin 2016;26:305-14. [Crossref] [PubMed]
- Kuppusamy MK, Hubka M, Felisky CD, et al. Evolving management strategies in esophageal perforation: surgeons using nonoperative techniques to improve outcomes. J Am Coll Surg 2011;213:164-71; discussion 171-2. [Crossref] [PubMed]
- van Boeckel PG, Sijbring A, Vleggaar FP, et al. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther 2011;33:1292-301. [Crossref] [PubMed]
- Biancari F, D'Andrea V, Paone R, et al. Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studies. World J Surg 2013;37:1051-9. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Dake M, et al. An Assessment of the Optimal Time for Removal of Esophageal Stents Used in the Treatment of an Esophageal Anastomotic Leak or Perforation. Ann Thorac Surg 2015;100:422-8. [Crossref] [PubMed]
- Kirschniak A, Kratt T, Stuker D, et al. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc 2007;66:162-7. [Crossref] [PubMed]
- Mennigen R, Senninger N, Laukoetter MG. Novel treatment options for perforations of the upper gastrointestinal tract: endoscopic vacuum therapy and over-the-scope clips. World J Gastroenterol 2014;20:7767-76. [Crossref] [PubMed]
- Kuehn F, Loske G, Schiffmann L, et al. Endoscopic vacuum therapy for various defects of the upper gastrointestinal tract. Surg Endosc 2017;31:3449-58. [Crossref] [PubMed]
- Wedemeyer J, Schneider A, Manns MP, et al. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc 2008;67:708-11. [Crossref] [PubMed]


