
Clinical nomogram for lymph node metastasis in pathological T1 esophageal squamous cell carcinoma: a multicenter retrospective study
Introduction
Esophageal cancer (EC) is a common digestive tract cancer. The morbidity and mortality due to EC rank 7th and 6th worldwide, respectively (1). In recent years, with the improvement of individuals’ awareness of the need to seek medical treatment, the improvement of dietary habits and the dissemination of cancer prevention knowledge, the incidence of EC in high-incidence areas has tended to decrease (2). Esophageal squamous cell carcinoma (ESCC) is the most common histological type of EC in China. The five-year survival rate for patients with ESCC diagnosed in an early stage is greater than 90.0% after curative treatment (3,4).
Radical esophagectomy and lymph node dissection are the gold standard of treatment. Owing to improvements in surgical instruments and technology, endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) can be performed in patients with early-stage ESCC (5,6). A retrospective study showed that the endoscopic resection rates for stage T1a and stage T1b EC patients were 53.0% and 20.9%, respectively (7). The incidence of lymph node metastasis (LNM) in pathological T1 (pT1) ESCC is 7.0–16.0% in the mucosa and 16.0–38.2% in the submucosa (6,8,9). The higher risk of LNM in submucosa limits the application of endoscopic resection. In addition, previous studies have reported that the lymph node status is the most important prognostic factor in early-stage ESCC (4,8,10). Therefore, an accurate prediction of the risk of LNM in T1 ESCC significantly affects treatment decisions and prognostic predictions.
A few studies have reported that LNM in stage T1 EC is related to the depth of tumor invasion, degree of tumor differentiation, tumor location and tumor size (5,8,9). However, there is still controversy. In addition, previous studies indicated that the preoperative alanine aminotransferase/aspartate aminotransferase ratio (LSR) and preoperative high-density lipoprotein cholesterol (HDL-C) level were factors affecting the prognosis of ESCC (11-13). However, no previous studies have proven their relationship with LNM. We speculated that some preoperative hematological indicators could also reflect the lymph node status. In addition, it is essential to construct an effective model for the prediction of the risk of LNM to select the optimal treatment and lymphadenectomy strategy for ESCC.
Methods
Patients
This study was a retrospective study of data from 243 patients with pT1 ESCC who underwent esophagectomy at the Affiliated Hospital of North Sichuan Medical College and Nanchong Central Hospital from February 2013 to June 2019. The following criteria were used for inclusion in this study: (I) patients with primary ESCC; (II) patients who underwent McKeown esophagectomy (thoracotomy/video-assisted thoracic surgery) and three-field lymphadenectomy; and (III) reevaluation of the postoperative pathology showed that the tumor only infiltrated the mucosal layer or the submucosa. The following exclusion criteria were used: (I) patients with esophagogastric junction carcinoma; (II) patients who received preoperative neoadjuvant therapy; (III) patients with distant metastases; (IV) patients with any concurrent primary cancer of other organs; and (V) patients >80 years old. The Ethics Committees and Review Board of the Affiliated Hospital of North Sichuan Medical College approved the study, and the need for patient consent was waived.
Patients were categorized into the negative group and the positive group according to whether there was LNM. The following variables were extracted from the database: sex, age, tumor location, degree of tumor differentiation, T1 sub-stage, tumor size, carcinoembryonic antigen (CEA) level, neutrophil count, lymphocyte count, LSR and HDL-C level.
This study based the tumor dissection, pathological staging, and lymph node status on the American Joint Committee on Cancer (AJCC) & The Union for International Cancer Control (UICC) 8th edition EC TNM classification criteria (14). The lymph node metastasis ratio (LNMR) was calculated as follows: (number of pathologically confirmed LNM/total number of lymph nodes dissected) ×100%.
Surgical procedures
All patients underwent gastroscopy, upper gastrointestinal radiography and contrast-enhanced computed tomography (CT) of the neck, chest, and upper abdomen prior to surgery. Esophageal mucosa staining was performed in patients with unclear lesions, and esophageal biopsy was performed to confirm the preoperative diagnosis. No preoperative neoadjuvant therapy was administered, and no contraindications for surgery were noted. All patients underwent McKeown esophagectomy with three-field lymphadenectomy.
Experienced pathologists completed the postoperative pathology reports. All specimens were analyzed for the depth of tumor invasion, degree of tumor differentiation, and the presence of lymphatic invasion. In patients with multifocal cancer, the lesion with the greatest invasion depth was chosen for the classification of tumor depth and the evaluation of lymph node status.
Statistical analysis
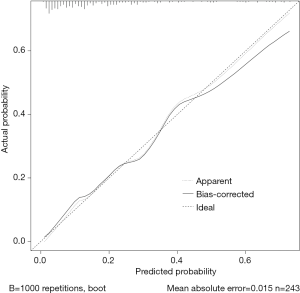
The statistical analysis was performed using IBM SPSS Statistics (version 22.0 Inc., Chicago, IL, USA) and the R programming language (version 3.4.1, Vienna, Austria). Data are reported as the frequencies, means and medians with percentages. The chi-square test and Student’s t-tests were performed in univariate analysis to determine the differences in parameters between the two groups. Factors found to be significant (P<0.050) in univariate analysis were included in the subsequent multivariate logistic regression analysis to identify the independent risk variables associated with LNM. The nomogram was constructed based on the results of the multivariate analysis and evaluated by the receiver operating characteristic (ROC) curve, the area under the ROC curve (AUC), and the calibration curve. The calibration curve was based on 1,000 bootstrap replicates (15). The odds ratio (OR) and 95% confidence interval (CI) were calculated. A P value <0.05 was considered statistically significant.
Results
Characteristics
A total of 243 patients were included in the analysis: 160 (65.8%) patients were male, and 83 (34.2%) patients were female. The median age was 64.6±7.59 years. The distribution of tumor locations in all patients was as follows: 41 (16.9%), 161 (66.2%) and 41 (16.9%) patients had EC in the upper, middle and lower esophagus, respectively. The distribution of the degree of tumor differentiation was as follows: 92 (37.9%), 130 (53.3%), and 21 (8.6%) had G1, G2 and G3 disease, respectively. The numbers of patients with stage T1a and T1b disease were 88 (36.2%) and 155 (63.8%), respectively. The mean tumor size, CEA level, neutrophil count, lymphocyte count, LSR and HDL-C level were 2.32±1.11 cm, 2.39±1.53 µg/L, 4.46±1.99 109/L, 1.63±0.57 109/L, 0.84±0.30 and 1.30±0.31 mmol/L, respectively. The clinicopathological and hematological characteristics of patients in the LNM-negative and LNM-positive groups are shown in Table 1.
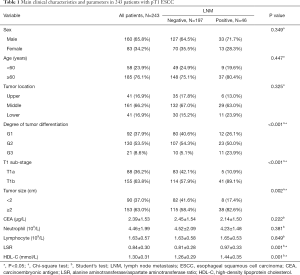
Full table
The prevalence of LNM
Forty-six (18.9%) of the 243 pT1 ESCC patients exhibited LNM. The LNM rates in patients with T1a and T1b disease were 5.7% (5/88) and 26.5% (41/155), respectively. A total of 6,240 lymph nodes were dissected during surgery, with a mean of 27±6 lymph nodes. Two hundred sixty-two lymph nodes were metastatic. The LNMR was 4.2% (262/6,240). The LNMRs in patients with T1a and T1b disease were 0.8% (16/1,999) and 5.8% (246/4,241), respectively.
The risk factors for LNM
The results of the univariate analysis revealed that the factors affecting LNM in T1 ESCC were the degree of tumor differentiation, T1 sub-stage, tumor size, LSR and HDL-C level (P<0.050). There was no significant difference in sex, age, tumor location, CEA level, neutrophil count or lymphocyte count (P=0.349, 0.447, 0.325, 0.053, 0.222, 0.381 and 0.849, respectively) (Table 1).
Multivariate logistic regression analysis demonstrated that the independent risk factors for LNM were tumor differentiation (OR =1.942, 95% CI: 1.067–3.536, P=0.030), the T1 sub-stage (OR =4.750, 95% CI: 1.658–13.611, P=0.004), the LSR (OR =5.371, 95% CI: 1.676–17.210, P=0.005), and the HDL-C level (OR =5.894, 95% CI: 1.917–18.124, P=0.002) (Table 2).
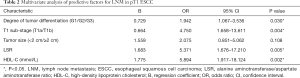
Full table
Nomogram
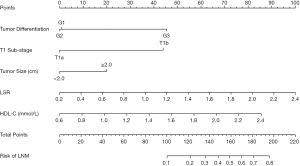
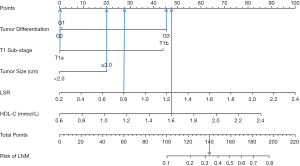
The established nomogram allowed for the estimation of the individual risk of LNM (Figure 1). A total score was calculated based on the degree of tumor differentiation, T1 sub-stage, tumor size, the LSR and the HDL-C level. A total score could be easily calculated by summing each individual score, and by projecting the total score to the lower total point scale, we were able to predict the probability of LNM. It also illustrated the relative contribution of each factor to the overall risk for LNM.
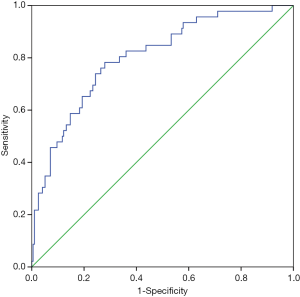
The ROC analysis is shown in Figure 2, which demonstrated that the nomogram had a robust discriminatory ability, with an AUC of 0.803 (95% CI: 0.732–0.873) (Figure 2). According to the calibration curve, the LNM probabilities predicted by the nomogram were consistent with the actual probabilities (Figure 3).
Discussion
Previous studies reported that the LNM rate in patients with T1a EC was 8.6–16.0%, and the rate in those with T1b was 16.0–34.3% (6,8,9,16). The differences in the incidences of LNM between reports may result from differences in the pathological type, sample size, method of lymph node dissection, and quality of the histopathological assessment of the resected samples (8).
The results of our study revealed an incidence of LNM of 5.7% (5/88) in stage T1a ESCC. This incidence was similar to that reported by Toshiaki et al. (17). The present study demonstrated an incidence of LNM of 26.5% (41/155) in stage T1b ESCC. A retrospective study of 295 patients who underwent surgery and/or ESD/EMR demonstrated that the T1b ESCC LNM rate was 34.3% (35/102) (16). This result may be partially attributed to the fact that both studies focused on the resection of lymph nodes and the evaluation of postoperative pathological sections, resulting in a higher LNM rate. However, Nentwich et al. (18) reported that the LNM rate in patients with T1b ESCC was 16.7% (5/30). The difference between these two results may be due to the larger sample size of our study and the fact that the patients underwent three-field lymphadenectomy.
Previous studies indicated that a worse degree of differentiation of ESCC resulted in a higher LNM rate. Our results showed that the LNM rates of G1, G2 and G3 tumors were 13.0% (12/92), 17.7% (23/130), and 52.4% (11/21), respectively. Shen et al. (19) reported that the LNM rates of G1, G2 and G3 tumors were 6.1% (3/49), 17.2% (17/99) and 45.2% (33/73) respectively, which were similar to the values obtained in our study. Akutsu et al. (16) reported that the LNM rates of well/moderately differentiated and poorly differentiated tumors were 17.4% (36/207) and 35.1% (13/37), respectively, which was different from our results of 15.8% (35/222) and 52.4% (11/21). There were fewer patients with poorly differentiated tumors in our study, but we still found that patients with G3 tumor differentiation had a significantly higher risk of LNM. We also found that the LNM rate of G3 tumors was 2-3 times higher than that of G1-G2 tumors.
The depth of tumor invasion (T1b) was one of the risk factors that affected LNM in the present study (P<0.05). The incidence of LNM increased markedly after the tumor invaded through the mucosal layer to the submucosa (16). Endoscopic treatment is acceptable for patients with limited LNM and stage T1a disease (5). However, whether it is suitable for patients with a high risk of LNM and stage T1b disease remains controversial. Previous studies reported that the submucosa was divided into sm1, sm2 and sm3, and the risk of LNM in each layer was assessed to confirm the application of endoscopic resection in patients with stage T1b disease (5,6,20). However, preoperative examinations are suitable for patients with stage T1a and T1b disease, and it is difficult to further differentiate the T1b sub-stage (10,18). Furthermore, the submucosa is a thin layer, and endoscopic resection has no absolute safety zone (9). The LNM rate of patients with disease extending into the submucosa in this study was 26.5%. There is a high risk of LNM when using endoscopic treatment in patients with stage T1b ESCC.
Tumor size is an important index that refers to the maximum diameter of the primary tumor, and it is easily measured before and during the operation (21). Duan et al. reported that a tumor size larger than 2.5 cm was a risk factor for LNM, and the LNM rates of tumors smaller than 2.5 cm and larger than 2.5 cm were 9.8% (8/82) and 27.9% (17/61), respectively (8). We used 2 cm as the threshold, and the results showed that the LNM rates of tumors smaller than 2 cm and larger than 2 cm were 8.9% (8/90) and 24.8% (38/153), respectively. The results were consistent despite the differences in tumor size thresholds. In our study, the chi-square test showed a statistically significant difference in tumor size between the LNM-negative and LNM-positive groups. However, multivariate regression analysis showed that a tumor size greater than 2 cm was not a risk factor for LNM, possibly due to the collinearity of the included indicators.
The alanine aminotransferase (ALT) level, aspartate aminotransferase (AST) level, and LSR are often used to assess liver damage, metabolic syndrome and cardiovascular disease (22). Previous studies have reported that patients with a high LSR have a good prognosis (12,13). The LSR may affect some proinflammatory mediators (e.g., CCL2, TNF, and IL-6) involved in carcinogenesis and tumor invasion and metastasis (13). One study reported that alcohol consumption and the AST/ALT ratio were independent risk factors for the incidence of EC in Korean men (23). However, whether the level of the LSR affects LNM in T1 ESCC has not been reported. Our results showed that patients with higher LSR exhibited a significantly higher risk of LNM. We hypothesized that the CCL1 in the lymphatic sinus is expressed in large amounts when tumor cells metastasize via the flow of the lymph, and the entry of tumor cells into the lymph nodes is controlled (24). The proinflammatory mediators TNF, IL-1β, and LPS increase CCL1 production and tumor cell migration to lymphatic endothelial cells (24). The level of the LSR affects the functions of proinflammatory factors and chemokines and indirectly affects LNM.
HDL-C is an antiatherosclerotic lipoprotein that is considered a protective factor against coronary heart disease (25). Previous studies reported that EC patients with low levels of HDL-C exhibited a poor prognosis and that the HDL-C levels were significantly decreased in patients with cancer compared to normal human blood lipid levels (11,26). The reason for the reduced HDL-C level in cancer is that growing cancer cells require a large amount of cholesterol to synthesize new cell membranes. The activity of the HDL-C receptor is increased, and the outflow of intracellular cholesterol is increased, which reduces the amount of HDL-C in the serum (27). No previous studies have reported the relationship between serum HDL-C levels and LNM in ESCC. However, in a study on LNM in gastric cancer, we found that a low HDL-C level was a risk factor for LNM (28), which was contrary to our findings that a high HDL-C level was a risk factor in ESCC. We suspected that in ESCC and gastric cancer, the mechanism may be somewhat different. Of course, more mechanism studies are needed to verify this conjecture.
In addition, our study developed a nomogram to estimate the probability of LNM in patients with T1 ESCC. In our nomogram, the specific probability of LNM was predicted, and the discriminatory ability and calibration were determined. A previous study (8) developed a nomogram to predict the risk of LNM in patients with pT1 ESCC but did not evaluate its discriminatory ability and calibration. The discriminatory ability of the nomogram was determined by the AUC. The predicted and actual probabilities of LNM were compared in a calibration diagram (19,29)). The AUC of our model was 0.803 (95% CI: 0.732–0.873), which proved that this model was highly accurate at predicting LNM. The calibration curve showed that the predicted probability of LNM was in good agreement with the actual probability. A total score was calculated from the 5 included parameters. The summarized total score indicates the probability of LNM. Figure 4 shows a patient with poor tumor differentiation (G3), invasion into the mucosal layer (pT1a), a 3 cm tumor (≥2.0 cm), an LSR of 0.8 and an HDL-C level of 1.6 mmol/L. For this patient, the calculated total score was 140=45+0+20+27.5+47.5, and the corresponding risk of LNM was 33%.
Limitations
Some inevitable limitations were present in our study. First, this study was a retrospective study with some selection bias. Second, our study found for the first time that the preoperative LSR and HDL-C level were independent risk factors for LNM. However, we have not identified the mechanism of these effect on LNM at the cellular and molecular levels, and further studies are needed. Third, our nomogram still needs to be validated in other databases due to the selected inclusion indicators and epidemiological differences.
Conclusions
Patients with pT1b ESCC exhibited a relatively high probability of LNM. The clinicopathological and hematological parameters of the degree of tumor differentiation, T1 sub-stage, preoperative LSR and HDL-C level may predict the risk of LNM in T1 ESCC. The risk of LNM in individuals can be predicted by the nomogram.
Acknowledgments
We appreciate the contribution and valuable assistance of Dr. Mao-Yong Fu and Dr. Lin Zhang of the Thoracic Surgery Department, Affiliated Hospital of North Sichuan Medical College. We would also like to thank the American Journal Experts (https://secure.aje.com/cn/researcher/) for editing the English text of a draft of this manuscript.
Funding: This work was supported by the Funds for Cooperation Project of Nanchong City and North Sichuan Medical College, Grant No. 18SXHZ0312 (to HYW); Key Subject of Affiliated Hospital of North Sichuan Medical College, Grant No. 2020ZD006 and Pre-research Project of North Sichuan Medical College No. CBY19-YZ19 (to DT); National Students’ Platform for Innovation and Intrepreneurship Training Program, Grant No. 201910634009 (to KYJ).
Footnote
Conflicts of Interest: DT serves as an unpaid Section Editor of Annals of Translational Medicine from Oct 2019 to Sep 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committees of the Affiliated Hospital of North Sichuan Medical College approved the study [No. 2018ER (R) 005] and the need for patient consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heymach J, Krilov L, Alberg A, et al. Clinical Cancer Advances 2018: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J Clin Oncol 2018;36:1020-44. [Crossref] [PubMed]
- Tian D, Mo SJ, Han LK, et al. Investigation of Dietary Factors and Esophageal Cancer Knowledge: Comparison of Rural Residents in High- and Low-incidence Areas. Sci Rep 2018;8:4914. [Crossref] [PubMed]
- Ning B, Abdelfatah MM, Othman MO. Endoscopic submucosal dissection and endoscopic mucosal resection for early stage esophageal cancer. Ann Cardiothorac Surg 2017;6:88-98. [Crossref] [PubMed]
- Minashi K, Nihei K, Mizusawa J, et al. Efficacy of Endoscopic Resection and Selective Chemoradiotherapy for Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology 2019;157:382-90.e3. [Crossref] [PubMed]
- Gamboa AM, Kim S, Force SD, et al. Treatment allocation in patients with early-stage esophageal adenocarcinoma: Prevalence and predictors of lymph node involvement. Cancer 2016;122:2150-7. [Crossref] [PubMed]
- Weksler B, Kennedy KF, Sullivan JL. Using the National Cancer Database to create a scoring system that identifies patients with early-stage esophageal cancer at risk for nodal metastases. J Thorac Cardiovasc Surg 2017;154:1787-93. [Crossref] [PubMed]
- Merkow RP, Bilimoria KY, Keswani RN, et al. Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst 2014;106:1-8. [Crossref] [PubMed]
- Duan XF, Tang P, Shang XB, et al. The prevalence of lymph node metastasis for pathological T1 esophageal cancer: a retrospective study of 143 cases. Surg Oncol 2018;27:1-6. [Crossref] [PubMed]
- Gertler R, Stein HJ, Schuster T, et al. Prevalence and topography of lymph node metastases in early esophageal and gastric cancer. Ann Surg 2014;259:96-101. [Crossref] [PubMed]
- Dubecz A, Kern M, Solymosi N, et al. Predictors of Lymph Node Metastasis in Surgically Resected T1 Esophageal Cancer. Ann Thorac Surg 2015;99:1879-85. [Crossref] [PubMed]
- Chen P, Han L, Wang C, et al. Preoperative serum lipids as prognostic predictors in esophageal squamous cell carcinoma patients with esophagectomy. Oncotarget 2017;8:41605-19. [PubMed]
- Chen SL, Li JP, Li LF, et al. Elevated Preoperative Serum Alanine Aminotransferase/Aspartate Aminotransferase (ALT/AST) Ratio Is Associated with Better Prognosis in Patients Undergoing Curative Treatment for Gastric Adenocarcinoma. Int J Mol Sci 2016. [Crossref] [PubMed]
- Huang H, Wang XP, Li XH, et al. Prognostic value of pretreatment serum alanine aminotransferase/aspartate aminotransferase (ALT/AST) ratio and gamma glutamyltransferase (GGT) in patients with esophageal squamous cell carcinoma. BMC Cancer 2017;17:544. [Crossref] [PubMed]
- Rice TW, Gress DM, Patil DT, et al. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:304-17.
- Liu Y, Zou ZQ, Xiao J, et al. A nomogram prediction model for recurrent laryngeal nerve lymph node metastasis in thoracic oesophageal squamous cell carcinoma. J Thorac Dis 2019;11:2868-77. [Crossref] [PubMed]
- Akutsu Y, Uesato M, Shuto K, et al. The overall prevalence of metastasis in T1 esophageal squamous cell carcinoma: a retrospective analysis of 295 patients. Ann Surg 2013;257:1032-8. [Crossref] [PubMed]
- Tanaka T, Matono S, Mori N, et al. T1 squamous cell carcinoma of the esophagus: long-term outcomes and prognostic factors after esophagectomy. Ann Surg Oncol 2014;21:932-8. [Crossref] [PubMed]
- Nentwich MF, von Loga K, Reeh M, et al. Depth of submucosal tumor infiltration and its relevance in lymphatic metastasis formation for T1b squamous cell and adenocarcinomas of the esophagus. J Gastrointest Surg 2014;18:242-9. [Crossref] [PubMed]
- Shen W, Shen Y, Tan L, et al. A nomogram for predicting lymph node metastasis in surgically resected T1 esophageal squamous cell carcinoma. J Thorac Dis 2018;10:4178-85. [Crossref] [PubMed]
- Manner H, Wetzka J, May A, et al. Early-stage adenocarcinoma of the esophagus with mid to deep submucosal invasion (pT1b sm2-3): the frequency of lymph-node metastasis depends on macroscopic and histological risk patterns. Dis Esophagus 2017;30:1-11. [PubMed]
- Zhang H, Tang P, Miao X, et al. Does tumor size improve the accuracy of prognostic prediction in patients with esophageal squamous cell carcinoma after surgical resection? Oncotarget 2016;7:66623-34. [PubMed]
- Oren R. Serum liver enzymes--should we count on them? Liver Int 2014;34:171-3. [Crossref] [PubMed]
- Kimm H, Kim S, Jee SHJYMJ. The Independent Effects of Cigarette Smoking, Alcohol Consumption, and Serum Aspartate Aminotransferase on the Alanine Aminotransferase Ratio in Korean Men for the Risk for Esophageal Cancer. 2010;51:310-7.
- Das S, Sarrou E, Podgrabinska S, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. 2013;210:1509.
- Liu YY, Lin SJ, Chen YY, et al. High-density lipoprotein cholesterol as a predictor of poor survival in patients with nasopharyngeal carcinoma. Oncotarget 2016;7:42978-87. [PubMed]
- Wang XP, Li XH, Zhang L, et al. High level of serum apolipoprotein A-I is a favorable prognostic factor for overall survival in esophageal squamous cell carcinoma. BMC Cancer 2016;16:516. [Crossref] [PubMed]
- Morin EE, Li XA, Schwendeman A. HDL in Endocrine Carcinomas: Biomarker, Drug Carrier, and Potential Therapeutic. Front Endocrinol (Lausanne) 2018;9:715. [Crossref] [PubMed]
- Fan G, Hu D, Peng F, et al. Different Risk Profiles for the Postsurgical Prognosis of Gastric Cancer Patients with Different Blood Types: The FIESTA Study. J Cancer 2018;9:2885-94. [Crossref] [PubMed]
- Semenkovich TR, Yan Y, Subramanian M, et al. A Clinical Nomogram for Predicting Node-positive Disease in Esophageal Cancer. Ann Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]


