
Mitochondrial metabolism and cancer metastasis
Introduction
The mitochondrion is critical for oxidative phosphorylation (OXPHOS), β-oxidation of fatty acids, tricarboxylic acid (TCA) cycle, calcium handling, proline synthesis, and heme biosynthesis. Hence, mitochondrial dysfunction, particularly in their metabolic activities, is the major cause of many diseases such as metabolic diseases, cancer, and neurodegenerative diseases, as well as the aging process (1-3). Alongside, mitochondria attracted more attention from a metabolic perspective based on two findings: mitochondrial metabolites can drive oncogenesis (4), and mitochondrial metabolic plasticity can adapt to serve bioenergetic or anabolic functions through mitochondrial circuitry changes (5,6). Thus, mitochondrial metabolism now constitutes a promising target for novel anticancer therapy (7).
Metastasis ranks as the first leading cause of cancer mortality worldwide. It is a complex process at the cellular level—only a few cells detached from a primary tumor can inflow into the blood vessels or lymphatic system, then successfully colonize a distant area in the body (8). As a prominent signal for cancer, mitochondrial dysfunction shows a significant correlation with poorer tumor progression and increased metastasis (9,10).
In this review, we address recent insights into the metabolic plasticity of cancer cells and the metabolic coupling between these cells and their tumor micro-environment (TME) in the process of cancer metastasis, as well as a possible mechanism. We also discuss the coupling of mitochondrial proline metabolism and extracellular matrix (ECM) in metastasis. More importantly, we emphasize the crosstalk between mitochondrial proline metabolism and ECM, and how mechano-environment, such as stiffness, might affect cancer progression.
Altered mitochondrial metabolism of cancer cell in metastasis
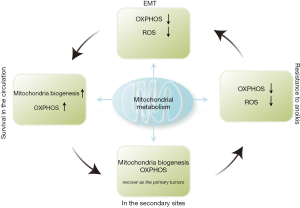
The metastatic cascade is a multi-step process, which includes (I) detachment of local tumor cells (resistance to anoikis), (II) intravasation (with the potential requirement of epithelial-to-mesenchymal transition, EMT), (III) survival in the blood circulation, (IV) extravasation from the circulation, and (V) colonization at the secondary sites (11-13). In all these stages, metastatic cells fine-tune their mitochondrial metabolism to better adapt to the ever-changing environment (13,14).
Mitochondrial metabolism on resistance to anoikis
During the metastasis, tumor cells are detached from the natural matrix niche. The first challenge for cells to metastasis is how to skip anoikis, which is programmed cell death that occurs in cells sensitive to matrix detachment. Unlike these normal cells, tumor cells have attained resistance to anoikis since many tumor cells already limit oxidative phosphorylation (OXPHOS) and reactive oxygen species (ROS) before detachment as a result of the Warburg effect (13) (Figure 1). Thus, tumor cells may inherently endow a survival advantage when detached from the primary tumor. Consistently, antioxidants can reduce oxidative stress and contribute to cancer progression in a lung cancer mouse model (15).
Mechanistically, pyruvate dehydrogenase kinases (PDKs), critical mitochondrial enzymes in glucose metabolism, could block OXPHOS and promote the Warburg effect (16). High expression of PDKs (such as PDK1) in tumor cells can evade excess ROS generated from glucose oxidation, which protects tumor cells from ROS-induced anoikis, thus promotes metastasis. On the contrary, loss of PDK in tumor cells can restore their susceptibility to anoikis owing to increased glucose oxidation and ROS production, leading to decreased metastatic potential (17). Consistently, Clinical analysis of human cancers reveals that PDKs expression in tumors has a strong positive correlation with the formation of distant metastases and a negative correlation with disease-free survival.
Besides, pyruvate kinase M2 (PKM2) is another important mitochondrial enzyme in the metabolic pathway of glucose (15), inhibition of which can shift glucose into pentose phosphate pathway, generating sufficient reducing potential for the detoxification of ROS. Meanwhile, the oxidative branch of the pentose phosphate pathway produces numerous NADPH (18,19), which is a critical cofactor for replenishing reduced glutathione (GSH), the most important antioxidant. Hence, increased antioxidant capacity also helps tumor cells to survive when they detach from the local sites.
Mitochondrial metabolism on EMT
Once tumor cells overcome the risk of anoikis, they activate the EMT process to help intravasate the blood or lymphatic vessel, inducing a metabolic rewiring (13). Metabolic reprogramming of tumor cells could be regulated by the EMT process through many molecules, including HIF, Snail, p53, and KISS1 (13). Among them, HIF promotes metastasis and suppresses oxidative metabolism as well as ROS generation. HIF-1 is an important regulator for glycolytic enzymes such as lactate dehydrogenase (LDH), hexokinase, and monocarboxylate transporter (MCT), hence favoring the glycolytic switch (20-22). Furthermore, HIF-1 also inhibits oxidative metabolism by upregulating PDK, an inhibitor of pyruvate dehydrogenase (PDH), which further prevent pyruvate from lowing into the TCA cycle (20,21,23). Another EMT inducer Snail regulates mitochondrial repression and ROS production by suppression of cytochrome C oxidase (COX) subunits or loss of fructose-1,6-bisphosphatase 1 (FBP1) (24). Involved in the first rate-limiting process of glycolysis, phosphofructokinase platelet (PFKP) can also be suppressed by Snail, which would shift the glucose flux more to the pentose phosphate pathway, leading to more NADPH production (24,25).
Meanwhile, p53 and KISS1 have been shown to suppress the Warburg effect and promote oxidative metabolism, resulting in blocking metastasis (26,27). P53 promotes the expression of OXPHOS through by upregulating of synthesis of cytochrome c oxidase (SCO2), which is a member of the COX-2 assembly and involved in oxidative metabolism as well as the electron-transport chain (28). KISS1 makes a metabolism shift from aerobic glycolysis to OXPHOS through stabilization of PGC1α, a key positive transcriptional regulator for metabolic genes in the TCA cycle and oxidative metabolism (29).
Emerging shreds of evidence suggest that the correlation between EMT and metabolism is mutual (14). Metabolic dysfunction can also drive EMT in some circumstances (14). Several mitochondrial metabolites favor the EMT (9,30), in particular, fumarate (31), succinate (32) and 2-hydroxyglutarate (2HG) (33), which are accumulated when mutation or deletion in fumarate hydratase (FH) (31,34-36), succinate dehydrogenase (SDH) (37-41), and isocitrate dehydrogenases (IDHs) (42-44) occur respectively. Mechanistically, Sciacovelli et al. reported that fumarate, succinate, and 2HG are responsible for the activation of EMT by suppressing the Ten-Eleven Translocation (TET)-dependent demethylation of miR200 (31), which is a known inhibitor of both SNAIL2 (45) and ZEB1 (31,46), thus showing anti-metastatic effect in breast cancer (33) and colorectal cancer (33,47). In addition, succinate accumulation promotes cell migration and invasion by activating the TGF-β/SNAIL1 pathway in colorectal cancer (48) and ovarian cancer (49); while in lung cancer and a human lung xenograft-mouse model, loss of SDH5 (50) induces EMT through activating the axis of glycogen-synthase kinase (GSK-3β)-β-catenin (51).
Mitochondrial metabolism on cell survival in the circulation
In order to survive in the blood or lymphatic vessel, mitochondrial circuitry of cancer cells changes for adapting to the changing environment. Gene expression profiling coupled with bioinformatic analyses by LeBleu et al. reveal that circulating cancer cells (CCC) from breast cancer primarily rely on OXPHOS and increased ATP production, compared to cancer cells from the primary tumors (PCC) (Figure 1). Further research reveals that transcription co-activator PGC-1α was a key factor in enhancing OXPHOS, oxygen consumption rate, and mitochondrial biogenesis. Overexpression of PGC-1α promotes mitochondrial OXPHOS and enhances the invasion of breast cancer cells. By contrast, suppression of PGC-1α in these cells inhibits their invasive potential and attenuates metastasis. Consistently, the expression of PGC-1α in human cancer tissue is positively correlated with tumor progression and metastasis (52).
Mitochondrial metabolism on colonization at the secondary sites
When the CCC arrive at and proliferate at the secondary sites, their mitochondrial metabolisms recover as the primary tumors. There are similar gene expression levels on OXPHOS and mitochondrial biogenesis between metastatic cancer cells (MCC) and CCC, suggesting that expression of these genes are recovered as CCC colonize at their metastasis sites (52). However, in some MCC, their gene expression levels are merely partially recovered compared with that in primary cancer cells, potentially as a result of a collective mixture of MCC at different metastatic stages (i.e., arrest, extravasation, migration and proliferation stages) (52) (Figure 1).
Metabolic coupling of tumor microenvironment cells (TME) and cancer cells in metastasis
TME is crucial for tumor progression and metastasis (53), which is composed of non-transformed stromal cells including fibroblasts, mesenchymal stem cells (MSC), endothelial cells, various inflammatory cells as well as the ECM. Cancer-associated fibroblasts (CAFs) serve as a prominent component of TME, which favors tumor metastasis. Several studies reveal that CAFs and cancer cells are metabolically coupled in many types of human tumors such as breast, prostate (54), and head & neck cancers as well as lymphomas. On one hand, cancer cells foster oxidative stress in surrounding CAFs, forcing CAFs to undergo autophagy/mitophagy as well as aerobic glycolysis. On the other hand, anabolic cancer cells that undergo oxidative mitochondrial metabolisms are supported by recycled nutrients from CAFs, such as lactate, ketones, and glutamine (55,56). Moreover, in breast cancer patients, these lactate- or ketone-induced gene signatures are associated with poor clinical outcomes (including metastasis, recurrence and poor overall survival) (57). Last but not least, the administration of lactate dramatically enhances the lung metastasis rate (>10-fold) in a xenograft model of breast cancer (MDA-MB-231 cells) (58).
Mechanistically, HIF1-alpha and NF-κB signaling are the master pathways that drive oxidative stress, then induce autophagy and senescence in CAFs (59-63). Moreover, caveolin-1 (Cav-1) and monocarboxylate transporter 4 (MCT4) are new biomarkers of this CAF phenotype. Loss of Cav-1 can induce high MCT4 expression in the stroma, which has been found to promote cancer cell migration and metastasis (64,65). A recent study reveals that exosomes play important roles in the metabolic coupling between CAFs and cancer cells. Exosomes, secreted by patient-derived CAFs, can strikingly induce metabolic reprogramming after their uptake by cancer cells, which inhibits mitochondrial OXPHOS, subsequently increases glycolysis and glutamine-dependent reductive carboxylation in prostate and pancreatic cancers (53).
Coupling of mitochondrial proline metabolism and ECM in metastasis
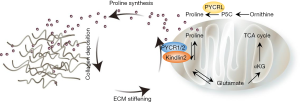
ECM is an intricate structural support network, which plays a positive role in the metastasis of cancer cells (66). Collagen is one of the most important components in the tumor ECM, especially in solid tumors. More importantly, collagen provides mechanical strength (67). Proline, a critical component of collagen, restricts the rotation of the polypeptide collagen chain by cyclic structure, hence strengthens the helical characteristic of the molecule (62,63). About 99.8% of hydroxyproline in the human body is stored in collagen and is an indicator of the total volume of collagen.
A new study reveals that mitochondrial proline metabolism and ECM are strongly correlated (68). Proline synthesis is strikingly influenced by the mechano-environment. For example, proline synthesis is decreased when cells are plated on soft ECM, while stiffening ECM significantly increases proline synthesis. In turn, alteration of proline metabolism may affect the collagen synthesis as well as ECM remodeling in lung cancer, since ~23% of the amino acid of the collagen molecule is comprised of proline and hydroxyproline (69-71), which are crucial for the biosynthesis, structure, and strength of collagen. Mechanistically, as an integrin-associated protein, kindlin-2 is well-known to localize at focal adhesions (72-79) sites where ECM proteins are linked to actin stress fibers (80). Interestingly, it is newly found to be translocated into mitochondria and interacts with pyrroline-5-carboxylate reductase 1 (PYCR1), which is a critical enzyme for proline metabolism in organisms. Furthermore, the stiffening of ECM promotes the translocation of Kindlin-2 as well as its interaction with PYCR1 in mitochondria, increasing the PYCR1 protein level, thus promoting proline synthesis and cell proliferation (Figure 2).
Acknowledgments
Funding: This work was supported by the Ministry of Science and Technology of China grant 2016YFC1302100, the National Natural Science of Foundation of China grants 81602472, and the Shenzhen Innovation Committee of Science and Technology grants JCYJ20180302174228311.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Khalid Sossey-Alaoui) for the Series “Cancer Metastasis: Molecular signaling and therapeutic options” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.42). The series “Cancer Metastasis: Molecular signaling and therapeutic options” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol 2016;212:379-87. [Crossref] [PubMed]
- Carelli V, Chan DC. Mitochondrial DNA: impacting central and peripheral nervous systems. Neuron 2014;84:1126-42. [Crossref] [PubMed]
- Lightowlers RN, Taylor RW, Turnbull DM. Mutations causing mitochondrial disease: What is new and what challenges remain? Science 2015;349:1494-9. [Crossref] [PubMed]
- Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009;462:739-44. [Crossref] [PubMed]
- Fendt SM, Bell EL, Keibler MA, et al. Reductive glutamine metabolism is a function of the alpha-ketoglutarate to citrate ratio in cells. Nat Commun 2013;4:2236. [Crossref] [PubMed]
- Wise DR, Ward PS, Shay JE, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A 2011;108:19611-6. [Crossref] [PubMed]
- Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, et al. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol 2017;14:11-31. [Crossref] [PubMed]
- Oskarsson T, Batlle E, Massague J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell 2014;14:306-21. [Crossref] [PubMed]
- Porporato PE, Filigheddu N, Pedro JMB, et al. Mitochondrial metabolism and cancer. Cell Res 2018;28:265-80. [Crossref] [PubMed]
- Lunetti P, Di Giacomo M, Vergara D, et al. Metabolic reprogramming in breast cancer results in distinct mitochondrial bioenergetics between luminal and basal subtypes. FEBS J 2019;286:688-709. [Crossref] [PubMed]
- van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 2011;728:23-34. [Crossref] [PubMed]
- Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med 2013;19:1450-64. [Crossref] [PubMed]
- Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett 2015;356:156-64. [Crossref] [PubMed]
- Sciacovelli M, Frezza C. Metabolic reprogramming and epithelial-to-mesenchymal transition in cancer. FEBS J 2017;284:3132-44. [Crossref] [PubMed]
- Anastasiou D, Poulogiannis G, Asara JM, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 2011;334:1278-83. [Crossref] [PubMed]
- Zhang W, Zhang SL, Hu X, et al. Targeting Tumor Metabolism for Cancer Treatment: Is Pyruvate Dehydrogenase Kinases (PDKs) a Viable Anticancer Target? Int J Biol Sci 2015;11:1390-400. [Crossref] [PubMed]
- Kamarajugadda S, Stemboroski L, Cai Q, et al. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol 2012;32:1893-907. [Crossref] [PubMed]
- DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv 2016;2:e1600200. [Crossref] [PubMed]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011;11:85-95. [Crossref] [PubMed]
- Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008;8:705-13. [Crossref] [PubMed]
- Meijer TW, Kaanders JH, Span PN, et al. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res 2012;18:5585-94. [Crossref] [PubMed]
- Singh D, Arora R, Kaur P, et al. Overexpression of hypoxia-inducible factor and metabolic pathways: possible targets of cancer. Cell Biosci 2017;7:62. [Crossref] [PubMed]
- Tello D, Balsa E, Acosta-Iborra B, et al. Induction of the mitochondrial NDUFA4L2 protein by HIF-1alpha decreases oxygen consumption by inhibiting Complex I activity. Cell Metab 2011;14:768-79. [Crossref] [PubMed]
- Kim NH, Cha YH, Lee J, et al. Snail reprograms glucose metabolism by repressing phosphofructokinase PFKP allowing cancer cell survival under metabolic stress. Nat Commun 2017;8:14374. [Crossref] [PubMed]
- Dong C, Yuan T, Wu Y, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 2013;23:316-31. [Crossref] [PubMed]
- Berkers CR, Maddocks OD, Cheung EC, et al. Metabolic regulation by p53 family members. Cell Metab 2013;18:617-33. [Crossref] [PubMed]
- Liu W, Beck BH, Vaidya KS, et al. Metastasis suppressor KISS1 seems to reverse the Warburg effect by enhancing mitochondrial biogenesis. Cancer Res 2014;74:954-63. [Crossref] [PubMed]
- Matoba S, Kang JG, Patino WD, et al. p53 regulates mitochondrial respiration. Science 2006;312:1650-3. [Crossref] [PubMed]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 2006;27:728-35. [Crossref] [PubMed]
- Frezza C. Mitochondrial metabolites: undercover signalling molecules. Interface Focus 2017;7:20160100. [Crossref] [PubMed]
- Sciacovelli M, Goncalves E, Johnson TI, et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature 2016;537:544-7. [Crossref] [PubMed]
- Loriot C, Burnichon N, Gadessaud N, et al. Epithelial to mesenchymal transition is activated in metastatic pheochromocytomas and paragangliomas caused by SDHB gene mutations. J Clin Endocrinol Metab 2012;97:E954-62. [Crossref] [PubMed]
- Grassian AR, Lin F, Barrett R, et al. Isocitrate dehydrogenase (IDH) mutations promote a reversible ZEB1/microRNA (miR)-200-dependent epithelial-mesenchymal transition (EMT). J Biol Chem 2012;287:42180-94. [Crossref] [PubMed]
- Kiuru M, Launonen V, Hietala M, et al. Familial cutaneous leiomyomatosis is a two-hit condition associated with renal cell cancer of characteristic histopathology. Am J Pathol 2001;159:825-9. [Crossref] [PubMed]
- Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 2002;30:406-10. [Crossref] [PubMed]
- He X, Yan B, Liu S, et al. Chromatin Remodeling Factor LSH Drives Cancer Progression by Suppressing the Activity of Fumarate Hydratase. Cancer Res 2016;76:5743-55. [Crossref] [PubMed]
- Maio N, Ghezzi D, Verrigni D, et al. Disease-Causing SDHAF1 Mutations Impair Transfer of Fe-S Clusters to SDHB. Cell Metab 2016;23:292-302. [Crossref] [PubMed]
- D'Antongiovanni V, Martinelli S, Richter S, et al. The microenvironment induces collective migration in SDHB-silenced mouse pheochromocytoma spheroids. Endocr Relat Cancer 2017;24:555-64. [Crossref] [PubMed]
- Huang Y, Wang LA, Xie Q, et al. Germline SDHB and SDHD mutations in pheochromocytoma and paraganglioma patients. Endocr Connect 2018;7:1217-25. [Crossref] [PubMed]
- Saxena N, Maio N, Crooks DR, et al. SDHB-Deficient Cancers: The Role of Mutations That Impair Iron Sulfur Cluster Delivery. J Natl Cancer Inst 2016;108:djv287. [Crossref] [PubMed]
- Bayley JP, van Minderhout I, Weiss MM, et al. Mutation analysis of SDHB and SDHC: novel germline mutations in sporadic head and neck paraganglioma and familial paraganglioma and/or pheochromocytoma. BMC Med Genet 2006;7:1. [Crossref] [PubMed]
- Sermer D, Pasqualucci L, Wendel HG, et al. Emerging epigenetic-modulating therapies in lymphoma. Nat Rev Clin Oncol 2019;16:494-507. [Crossref] [PubMed]
- Guo C, Pirozzi CJ, Lopez GY, et al. Isocitrate dehydrogenase mutations in gliomas: mechanisms, biomarkers and therapeutic target. Curr Opin Neurol 2011;24:648-52. [Crossref] [PubMed]
- Tommasini-Ghelfi S, Murnan K, Kouri FM, et al. Cancer-associated mutation and beyond: The emerging biology of isocitrate dehydrogenases in human disease. Sci Adv 2019;5:eaaw4543.
- Liu YN, Yin JJ, Abou-Kheir W, et al. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene 2013;32:296-306. [Crossref] [PubMed]
- Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10:593-601. [Crossref] [PubMed]
- Colvin H, Nishida N, Konno M, et al. Oncometabolite D-2-Hydroxyglurate Directly Induces Epithelial-Mesenchymal Transition and is Associated with Distant Metastasis in Colorectal Cancer. Sci Rep 2016;6:36289. [Crossref] [PubMed]
- Wang H, Chen Y, Wu G. SDHB deficiency promotes TGFbeta-mediated invasion and metastasis of colorectal cancer through transcriptional repression complex SNAIL1-SMAD3/4. Transl Oncol 2016;9:512-20. [Crossref] [PubMed]
- Aspuria PP, Lunt SY, Varemo L, et al. Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism. Cancer Metab 2014;2:21. [Crossref] [PubMed]
- Hao HX, Khalimonchuk O, Schraders M, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science 2009;325:1139-42. [Crossref] [PubMed]
- Liu J, Gao L, Zhang H, et al. Succinate dehydrogenase 5 (SDH5) regulates glycogen synthase kinase 3beta-beta-catenin-mediated lung cancer metastasis. J Biol Chem 2013;288:29965-73. [Crossref] [PubMed]
- LeBleu VS, O'Connell JT, Gonzalez Herrera KN, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 2014;16:992-1003, 1-15.
- Zhao H, Yang L, Baddour J, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife 2016;5:e10250. [Crossref] [PubMed]
- Fiaschi T, Marini A, Giannoni E, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res 2012;72:5130-40. [Crossref] [PubMed]
- Martinez-Outschoorn UE, Trimmer C, Lin Z, et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microenvironment. Cell Cycle 2010;9:3515-33. [Crossref] [PubMed]
- Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 2010;9:3256-76. [Crossref] [PubMed]
- Martinez-Outschoorn UE, Prisco M, Ertel A, et al. Ketones and lactate increase cancer cell "stemness," driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via Metabolo-Genomics. Cell Cycle 2011;10:1271-86. [Crossref] [PubMed]
- Bonuccelli G, Tsirigos A, Whitaker-Menezes D, et al. Ketones and lactate "fuel" tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 2010;9:3506-14. [Crossref] [PubMed]
- Witkiewicz AK, Kline J, Queenan M, et al. Molecular profiling of a lethal tumor microenvironment, as defined by stromal caveolin-1 status in breast cancers. Cell Cycle 2011;10:1794-809. [Crossref] [PubMed]
- Martinez-Outschoorn UE, Whitaker-Menezes D, Lin Z, et al. Cytokine production and inflammation drive autophagy in the tumor microenvironment: role of stromal caveolin-1 as a key regulator. Cell Cycle 2011;10:1784-93. [Crossref] [PubMed]
- Mazure NM, Pouyssegur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol 2010;22:177-80. [Crossref] [PubMed]
- Messadi DV, Doung HS, Zhang Q, et al. Activation of NFkappaB signal pathways in keloid fibroblasts. Arch Dermatol Res 2004;296:125-33. [Crossref] [PubMed]
- Mazure NM, Pouyssegur J. Atypical BH3-domains of BNIP3 and BNIP3L lead to autophagy in hypoxia. Autophagy 2009;5:868-9. [Crossref] [PubMed]
- Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, et al. Evidence for a stromal-epithelial "lactate shuttle" in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle 2011;10:1772-83. [Crossref] [PubMed]
- Martins D, Beca FF, Sousa B, et al. Loss of caveolin-1 and gain of MCT4 expression in the tumor stroma: key events in the progression from an in situ to an invasive breast carcinoma. Cell Cycle 2013;12:2684-90. [Crossref] [PubMed]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer 2001;1:46-54. [Crossref] [PubMed]
- Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 2011;209:139-51. [Crossref] [PubMed]
- Guo L, Cui C, Zhang K, et al. Kindlin-2 links mechano-environment to proline synthesis and tumor growth. Nat Commun 2019;10:845. [Crossref] [PubMed]
- Albaugh VL, Mukherjee K, Barbul A. Proline Precursors and Collagen Synthesis: Biochemical Challenges of Nutrient Supplementation and Wound Healing. J Nutr 2017;147:2011-7. [PubMed]
- Grant ME, Prockop DJ. The biosynthesis of collagen. 3. N Engl J Med 1972;286:291-300. [Crossref] [PubMed]
- Krane SM. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids 2008;35:703-10. [Crossref] [PubMed]
- Tu Y, Wu S, Shi X, et al. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 2003;113:37-47. [Crossref] [PubMed]
- Larjava H, Plow EF, Wu C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep 2008;9:1203-8. [Crossref] [PubMed]
- Montanez E, Ussar S, Schifferer M, et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev 2008;22:1325-30. [Crossref] [PubMed]
- Rognoni E, Ruppert R, Fassler R. The kindlin family: functions, signaling properties and implications for human disease. J Cell Sci 2016;129:17-27. [Crossref] [PubMed]
- Plow EF, Qin J, Byzova T. Kindling the flame of integrin activation and function with kindlins. Curr Opin Hematol 2009;16:323-8. [Crossref] [PubMed]
- Karakose E, Schiller HB, Fassler R. The kindlins at a glance. J Cell Sci 2010;123:2353-6. [Crossref] [PubMed]
- Wu C, Jiao H, Lai Y, et al. Kindlin-2 controls TGF-beta signalling and Sox9 expression to regulate chondrogenesis. Nat Commun 2015;6:7531. [Crossref] [PubMed]
- Guo L, Cai T, Chen K, et al. Kindlin-2 regulates mesenchymal stem cell differentiation through control of YAP1/TAZ. J Cell Biol 2018;217:1431-51. [Crossref] [PubMed]
- Burridge K. Focal adhesions: a personal perspective on a half century of progress. FEBS J 2017;284:3355-61. [Crossref] [PubMed]


