
Initial experience of a MitraClip valve repair program in Spain
Introduction
Mitral regurgitation (MR) is the most common left-side valve disease and its prevalence is rising especially in the elderly population (1). Two main etiologies have been described: primary/degenerative/organic MR (DMR) and secondary/functional MR (FMR). Primary MR is defined by the affectation of the components of the mitral apparatus (leaflets, chordae, papillary muscles). In FMR, there is integrity of the valve but geometric disturbances in the left ventricle or in the atrium are present and cause lack of coaptation of the mitral leaflets and usually mitral annulus dilatation. When the mechanism is a combination of DMR and FMR, this entity is defined as mixed MR (MMR) (2).
During its natural history, when severe MR becomes symptomatic the prognosis is impaired, as patients have elevated rates of mortality, worse clinical outcomes and reduced quality of life (3,4). In DMR, mitral valve repair or mitral valve replacement is recommended. Percutaneous mitral valve repair can be indicated in high risk patients (5). In FMR, surgical treatment is indicated when concomitant coronary revascularization is feasible. The isolated surgical treatment of FMR is controversial, without strong evidence in survival improvement or reduction of heart failure (HF) readmissions. In such scenario, the therapeutic options have been limited to medical treatment and cardiac resynchronization therapy (5,6). Transcatheter mitral valve repair (TMVR) with the MitraClip system (Abbott, Menlo Park, California, USA) has emerged as a feasible and safe strategy in reduction the severity of FMR with high rates of success and at least similar long-term outcomes to surgical repair, improving functional class and quality of life (7-9). Recently, two large randomized controlled trials have been published, that compare TMVR plus medical therapy vs. medical therapy alone, with relevant differences among the population included and results. The COAPT trial showed a significant reduction in the rates of hospitalization for HF and death from any cause in the group of TMVR, whereas MITRA-FR failed to show significant differences (10,11). In this regard, TMVR could be useful in combination with optimal medical treatment in selected patients with high degrees of MR severity and less advanced left ventricular disease.
The main objective of this study was to evaluate one year clinical outcome of patients with MR treated with TMVR in a high-volume center, according to the presence of primary, secondary or mixed types of MR.
Methods
Data from a single high-volume center of all consecutive cases with symptomatic MR undergoing TMVR where prospectively included in the study and followed. Each case was evaluated by a multidisciplinary heart team and selected this therapy. Informed consent was obtained from all patients.
Definitions
For the purpose of the investigation, three groups where defined according to the etiology of MR. Primary or degenerative or organic MR (DMR) was defined according to the presence of structural damage to one of the components of the mitral apparatus. Secondary or functional MR (FMR) was defined in the absence of structural damage to the valve but a disruption in the papillary muscles and chordae tendineae leading to impaired coaptation of the leaflets secondary to geometric disturbances in the left ventricle. And mixed MR (MMR) when there was a combination of both mechanisms (5,12). Bleeding events were recorded according to VARC II definition (13).
Procedural success was defined as a correct release of at least one device with a significative MR reduction reaching grade 2+ or less.
Follow-up
Patients where followed up at 3 months and at 1 year, with clinical and echocardiographic evaluations. There were no loses reported.
TMVR procedure
TMVR was performed with MitraClip edge to edge technique (Abbott, Menlo Park, California, USA) a cobalt-chromium two arm device that opens and closes through a special delivery system. It is advanced through a catheter into the left atrium via transseptal puncture. Once the clip is opened, it is aligned over the regurgitant jet with perpendicular orientation with the coaptation plane. The clip is advanced into the left ventricle and there after it is pulled in order to catch de leaflets. When the clip is closed it grasps the mitral leaflets effectively creating a double orifice valve. The procedures were performed under general anesthesia and guiding of the system with fluoroscopy and transesophageal echocardiography (TEE). Once the clip was positioned TEE assessment (3D and X-plane) was fundamental to confirm significant reduction of the MR and absence of mitral stenosis (MS). If the attempt was unsuccessful the clip was reopened and repositioned or a second device is attempted (14).
In our early experience we begun the TMVR program with the first generation of the device, and later we have used the second and third generation devices.
Endpoints
The primary endpoint was a composite of all-cause death and unplanned hear failure hospitalizations during the first year of follow-up. Secondary outcomes were the improvement in functional class according to the New York Heart Association (NYHA) and the reduction in the severity of MR after TMVR.
Statistical analysis
Statistical analysis was performed using Stata 15.2 (Stata Corp. LP, USA). Normal distribution for quantitative variables were assessed by the Kolmogorov-Smirnov test. Quantitative variables are expressed as mean ± standard deviation (SD) in case of normal distribution and median, 25th to 75th interquartile range (IQR) otherwise. Categorical variables are expressed as numbers (percentages). To analyze the primary endpoint time to event curves were calculated using Kaplan-Meier curves. Univariable Cox proportional hazard model was used to identify the factors associated with the cumulative primary endpoint calculating the HR with its 95% CI. P value <0.05 was considered statistically significant.
Results
Baseline characteristics
Between October 2015 and October 2019, a total of 81 consecutive patients underwent TMVR and were included in the investigation. The mean age was 75.73±7.81 years, 55 (67.9%) were male. The most frequent mechanism of MR was FMR (59%) followed by DMR (21%) and MMR (20%). The mean EuroSCORE II was 5.7±4.94 (FMR 5.38±3.9, DMR 5.72±4.7 and MMR 6.6±7.5; P=0.7776) and STS score mean was 5.21±3.31 (FMR 4.6±2.3, DMR 6.43±5.2 and MMR 5.7±3.2; P=0.126). There were no significant differences among the groups. Patients with FMR had higher rates of dilated (36 patients, 75.5%) and ischemic (15 patients, 31.3%) cardiomyopathy, as well as worse LVEF, with 23 (47.9%) patients with left ejection fraction below 35%. There were no significant differences in the rest of echocardiographic findings. Detailed baseline characteristics are shown in Table 1.
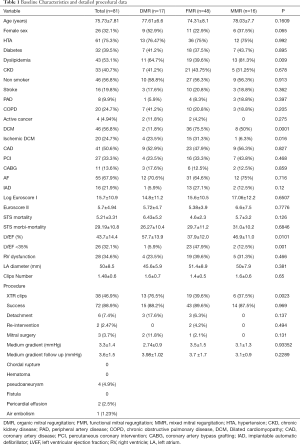
Full table
Procedural variables
Procedural data are detailed in Table 1. All procedures were elective and procedural success was achieved in 72 (88.9%) patients, with a similar distribution between the different etiologies. Procedural time and the number of implanted clips were similar among the three groups. In the follow-up, 6 (7.4%) cases of detachment were detected with 2 cases undergoing a second TMVR procedure and 4 cases referred for surgery.
A low rate of in-hospital complications was observed: 4 (4.9%) femoral pseudoaneurysms and 2 cases (2.5%) of mild pericardial effusion without hemodynamic compromise. There were no cases of bleeding or deaths before discharge.
Primary endpoint
The median of follow-up was 16.3 months (IQR, 5.75–26.15 months). The primary combined endpoint occurred in 19 (23.5%) cases. The number of events regarding the different etiologies were 15 (31.2%) in FMR, 2 (11.8%) in DMR and 5 (31.3%) in MMR with no significant differences amongst the groups (P=0.276) (Table 2).

Full table
Sixteen patients (20.0%) died during the first year of follow-up and 19 (23.5%) had unplanned HF hospitalization. There were no statistical differences between groups (Table 3). The Kaplan-Meier curves are shown in Figure 1A.
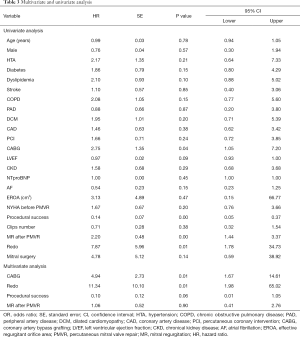
Full table
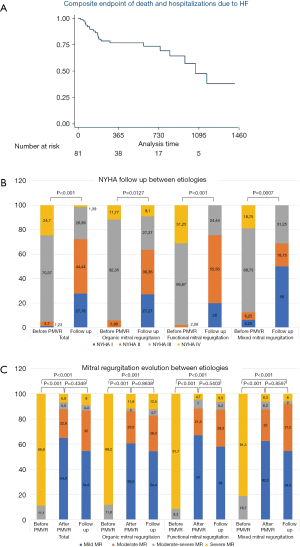
In the univariate analysis, the variables associated with the primary combined event were previous surgical revascularization (CABG), a redo TMVR procedure and MR after TMVR. In the multivariate analysis, previous CABG (HR =4.94, P=0.004) and a redo TMVR (HR =11.34, P=0.006) predicted the main event. Uni and multivariable analysis are shown in Table 3.
Secondary endpoints
Improvement in functional class
Seventy-seven (95.1%) patients had NYHA functional class ≥ III before TMVR, without differences between etiologies (P=0.25). The evolution of NYHA class is depicted in Figure 1B. There was an improvement of the functional class during the follow-up in all groups, with most of the patients (39.5%) being in NYHA stage II after 1 year of follow-up.
Reduction in MR severity
There was a significant reduction of the severity of MR among before and after TMVR. After TMVR most of the patients (87.6%) had grade 2 or less MR. This improvement was maintained through time (86.6% of the patients with MR ≤ II) after 12 months of follow-up. There were no significant differences between groups of different etiologies. Details are shown in Figure 1C.
Discussion
The present study was designed to describe the initial experience in a single high-volume center of patients with severe MR treated with the MitraClip device, including one-year follow-up clinical outcomes. The main findings were the high rate of procedural success, with an overall low rate of complications during hospitalization and after one year of follow-up. Moreover, the majority of patients were free of three/four degree of MR and with an improvement in their NYHA functional class.
The MitraClip device was approved in 2013 for the treatment of primary MR in patients with prohibitive risk for surgery. However, in this series 59% of the patients were treated for functional MR and had higher rates of dilated and ischemic cardiomyopathy with worse LVEF. These findings concur to other large European registries such as the ACCESS-EU (77.1%) the TRAMI (71%), the Spanish Mitraclip registry (65.2%) and the TCVT Registry (72%) and reflect the tendency to use this device for the treatment of functional MR in patients with HF (7,15-17).
In the present study, procedural success was achieved in 88.9% of the patients. Similar results were reported in the Everest II trial (90.5%). Furthermore, a meta-analysis by Mendirichaga et al. reported an acute device success of 89% (18,19). However, in more recent registries there have been even higher rates of success exceeding 95% in some cases (7,15-17). These results reflect the learning curve of TVMR, as most cases of device failure happened in the early stages of this series. There was a low rate of intraprocedural and in-hospital complications, with no bleedings according to BARC definitions and no deaths before discharge. These results are in line with other recent studies. Matsumoto et al. had no cases of emergent cardiac surgery and death during the procedure or subsequent hospital stay. Likewise, Giannini et al. and Capodanno et al. had no cases of mortality during the procedure (20,21).
After one year of follow-up 75.5% of the patients of this series were free of the primary combined endpoint and there were no significant differences amongst the groups (P=0.276). The Spanish MitraClip registry and the Sentinel registry showed similar results (81.1%). However, FMR has shown a tendency in several registries to worsen clinical outcomes (7,17). Such tendencies may be statistically significant provided a larger sample size and a longer follow-up. In the univariate and multivariate analysis there were no predictors of the main event.
All-cause mortality has shown some variations between European registries after one year of follow-up. The TRAMI and the ACCESS-EU trial had similar mortality rates to this study (20.3% and 19.2% respectively), whereas the GRASP and the Sentinel registries where lower (15.3% and 14.4% respectively). The higher mortality rate could be explained by the advance NYHA stage in most patients and a large representation of FMR secondary to ischemic and dilated cardiomyopathy, with a higher quantity of comorbidities. There were only 2 (2.47%) cases or re-intervention at follow-up. Similar results have been reported ranging from 1.5–2.8% (8,16,20). These results suggest TMVR as a durable solution for the treatment of MR.
Nowadays, MitraClip could play a role for symptomatic patients despite adequate medical therapy. In our study patients presented with a NYHA III–IV at baseline improving to a NYHA I-II functional class after PMVR in 68.3% the cases and with no cases in stage IV. Furthermore, after the baseline procedure 82.7% were free of severe MR explaining the symptomatic improvement over time.
Limitations
The main limitation of the present study is the relatively low sample size, single-center experience and observational nature of the investigation.
Conclusions
In conclusion, TMVR with the Mitraclip device is a safe procedure, with a low incidence of complications and a high rate of procedural success, similar to those reported in previous registries with similar scenarios. One-year outcomes show reduction of the primary combined endpoint (all cause death and hospitalizations due to HF). Moreover, most of the patients have sustained MR reduction and an improvement in the functional class at the end of follow-up.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Daniel Hernández-Vaquero) for the series “Structural Heart Disease: The Revolution” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.121). The series “Structural Heart Disease: The Revolution” was commissioned by the editorial office without any funding or sponsorship. DHV served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Translational Medicine from Aug 2019 to Jul 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the corresponding IRB (reference number: 2020/026). Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol 2014;30:962-70. [Crossref] [PubMed]
- Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: Pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231-48. [Crossref] [PubMed]
- Goliasch G, Bartko PE, Pavo N, et al. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2018;39:39-46. [Crossref] [PubMed]
- Sannino A, Smith RL, Schiattarella GG, Trimarco B, Esposito G, Grayburn PA. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: A systematic review and meta-analysis. JAMA Cardiol 2017;2:1130-9. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Rev Esp Cardiol (Engl Ed) 2018;71:110. [Crossref] [PubMed]
- Stone GW, Vahanian AS, Adams DH, et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 1: Clinical Trial Design Principles A Consensus Document from the Mitral Valve Academic Research Consortium. J Am Coll Cardiol 2015;66:278-307. [Crossref] [PubMed]
- Nickenig G, Estevez-Loureiro R, Franzen O, et al. Percutaneous mitral valve edge-to-edge Repair: In-hospital results and 1-year follow-up of 628 patients of the 2011-2012 pilot European Sentinel Registry. J Am Coll Cardiol 2014;64:875-84. [Crossref] [PubMed]
- Glower DD, Kar S, Trento A, et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: Results of the EVEREST II study. J Am Coll Cardiol 2014;64:172-81. [Crossref] [PubMed]
- Baldus S, Schillinger W, Franzen O, et al. Mitra Clip therapy in daily clinical practice: Initial results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2012;14:1050-5. [Crossref] [PubMed]
- Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med 2018;379:2297-306. [Crossref] [PubMed]
- Stone GW, Lindenfeld JA, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307-18. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document†. Eur Heart J 2012;33:2403-18. [Crossref] [PubMed]
- Feldman T, Foster E, Glower DD, et al. Percutaneous Repair or Surgery for Mitral Regurgitation. N Engl J Med 2011;364:1395-406. [Crossref] [PubMed]
- Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: Early and 1-year results from the ACCESS-EU, A prospective, multicenter, nonrandomized post-approval study of the Mitraclip therapy in Europe. J Am Coll Cardiol 2013;62:1052-61. [Crossref] [PubMed]
- Puls M, Lubos E, Boekstegers P, et al. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: Results from the German transcatheter mitral valve interventions registry. Eur Heart J 2016;37:703-12. [Crossref] [PubMed]
- Pascual I, Arzamendi D, Carrasco-Chinchilla F, et al. Transcatheter mitral repair according to the cause of mitral regurgitation: Real-life data from the Spanish MitraClip registry. Rev Esp Cardiol (Engl Ed) 2020;73:643-51. [Crossref] [PubMed]
- Feldman T, Kar S, Elmariah S, et al. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation 5-Year Results of EVEREST II. J Am Coll Cardiol 2015;66:2844-54. [Crossref] [PubMed]
- Mendirichaga R, Singh V, Blumer V, et al. Transcatheter Mitral Valve Repair With MitraClip for Symptomatic Functional Mitral Valve Regurgitation. Am J Cardiol 2017;120:708-15. [Crossref] [PubMed]
- Giannini C, Fiorelli F, De Carlo M, et al. Comparison of percutaneous mitral valve repair versus conservative treatment in severe functional mitral regurgitation. Am J Cardiol 2016;117:271-7. [Crossref] [PubMed]
- Capodanno D, Adamo M, Barbanti M, et al. Predictors of clinical outcomes after edge-to-edge percutaneous mitral valve repair. Am Heart J 2015;170:187-95. [Crossref] [PubMed]


