Novel targeted therapies for metastatic breast cancer
Introduction
Worldwide, breast cancer (BC) is the most common cancer in women and the second leading cause of cancer-related death in women in the United States. Despite an improvement in mortality trends, the American Cancer Society estimates that more than 42,000 patients will die of BC in the US in the year 2019, and the 5-year survival rate of metastatic BC (mBC) is at 27% (1). This indicates that we still have a way to go when it comes to the treatment of advanced BC.
mBC can be divided into three therapeutic subtypes: estrogen-receptor (ER) positive, HER-2 positive, and triple-negative BC (TNBC). ER-positive/HER-2 negative BC accounts for about 70% of BC cases (2), and traditionally these have been treated with endocrine therapy like tamoxifen and aromatase inhibitors. HER-2 positive cancers account for about 15–20% of the cases (3) and are classically treated anti-HER-2 agents such as trastuzumab, and TNBC accounts for about up to 15% of cases (4), with chemotherapy being the cornerstone of its treatment.
In recent years there have been new therapies developed and researched for the management of patients with mBC. We will review these novel treatment options available for each type of advanced BC and those that are still under investigation.
ER-positive BC
CDK4/6 inhibitors
CDK4 and CDK6 are proteins that play an important role in cellular proliferation and are often dysregulated or overexpressed in BCs, particularly HR-positive BCs (5,6). Inhibiting CDK4 and CDK6 in cancer cell cultures reduced the growth of cells in vitro, particularly among HR-positive BC cultures (7,8). This has translated to in vivo efficacy as numerous clinical trials have proven the utility of agents antagonizing the CDK4/6 pathway in ER-positive BC patients.
The main CDK4/6 inhibitors are palbociclib, ribociclib, and abemaciclib, and they are typically used in conjunction with endocrine therapy such as letrozole or fulvestrant. In the PALOMA-1/TRIO-18 trial, a phase 2 randomized control trial (RCT) done in patients with metastatic ER-positive BC, patients were randomized into groups receiving the aromatase inhibitor letrozole or letrozole and palbociclib. In the group receiving palbociclib, progression-free survival (PFS) was improved by 10 months [20.2 vs. 10.2 months; hazard ratio (HR) 0.49, 95% confidence interval (CI), 0.32–0.75; P<0.001] (9). The results of this trial led to the accelerated approval of palbociclib in the United States. The PALOMA-2 trial was a phase 3 trial that produced a similar 10-month improvement in PFS among patients taking the combination of letrozole and palbociclib versus letrozole alone (24.8 vs. 14.5 months; HR 0.58; 95% CI, 0.46–0.72; P<0.001) (10).
The PALOMA-3 trial was also conducted—a phase 3 trial to assess the benefit of palbociclib after metastatic ER-positive BC had progressed on endocrine therapy, to determine if CDK4/6 inhibitors have any role in overcoming resistance to anti-hormonal therapy. Patients received either fulvestrant and palbociclib or fulvestrant and placebo, and the group that received palbociclib had an improvement in their median PFS by almost 6 months (9.2 vs. 3.8 months; HR 0.42, 95% CI, 0.32–0.56; P<0.001) (11).
Ribociclib was also studied in combination with letrozole and produced similar results (12), with a PFS that was significantly longer than the placebo group (HR 0.56; 95% CI, 0.43–0.72; P<0.01). In the MONALEESA-7 trial, ribociclib in combination with endocrine therapy was shown to increase overall survival at 42 months when compared to placebo (70.2% vs. 46.0%, HR for death 0.71; 95% CI, 0.54–0.95; P<0.01) (13). Abemaciclib was studied in combination with fulvestrant in patients with metastatic ER-positive BC who had progressed on endocrine therapy, and the addition of abemaciclib to fulvestrant significantly improved median PFS when compared to fulvestrant alone (16.4 vs. 9.3 months; HR 0.55; 95% CI, 0.45–0.68; P<0.001) (14). The median OS of the abemaciclib group was 46.7 months compared to 37.3 months for the placebo group (HR 0.757; 95% CI, 0.606–0.945; P=0.01) (15). When used as first-line therapy for metastatic ER-positive BC, abemaciclib combined with an aromatase inhibitor also had improved PFS compared to an aromatase inhibitor alone (16).
Given the findings of these trials, adding CDK4/6 inhibitors to endocrine therapy is now standard of care for patients with metastatic ER-positive BC. Of note, the most common adverse events of the CDK4/6 inhibitors in these trials were neutropenia (20–60%) and leukopenia (7–21%) with ribociclib, and diarrhea (10–80%) and transaminitis (30–50%) with abemaciclib, and fatigue (up to 40%). Notably, ribociclib can prolong the QTc interval. As of yet, there are no biomarkers that are clinically useful to determine which patients will respond better to CDK4/6 inhibitors.
PI3K/AKT inhibitors
AKT is a serine/threonine kinase that interacts with phosphoinositides, which comprise 10-15% of membrane phospholipids, to produce several downstream effects that promote cell growth and proliferation. It is a part of the critical PI3K/AKT/mTOR pathway that is known to be a key mechanism of oncogenesis (17). PI3K mutations are frequently seen in BC that is ER-positive and is seen less often in ER-negative BCs, with the exception of certain subtypes of TNBC (18). This is likely due to the downstream effect of PI3K activation leading to the expression of the estrogen receptor and is the rationale behind combining PI3K inhibitors with endocrine agents.
The two major categories of PI3K inhibitors are the pan-class PI3K inhibitors such as buparlisib versus isoform-specific PI3K inhibitors such as alpelisib that are designed to be selective to one or more of the isoforms of the catalytic subunit of PI3K. Phase 1 dose-escalation studies of buparlisib showed that treatment was generally tolerated in patients with solid tumors (19,20). The phase 3 randomized BELLE-2 trial showed the addition of buparlisib plus fulvestrant versus placebo plus fulvestrant significantly improved PFS in advanced ER-positive/HER-2 negative BC (21), but the difference in OS was not statistically significant (22). Notably, there was more toxicity among the patients receiving buparlisib, particularly transaminitis, hyperglycemia, and rashes.
There has been a shift towards the use of isoform-specific PI3K inhibitors due to the theory of improved efficacy and reduced toxicity with more specific inhibition. The isoform-specific PI3K inhibitors target one or more of the four isoforms of the catalytic subunit of PI3K. In a phase Ib study of alpelisib combined with letrozole in ER-positive mBC, clinical benefit was seen in 35% of the 26 patients studied with objective responses in five patients (23). In the phase 3 SOLAR-1 trial, alpelisib combined with fulvestrant compared to fulvestrant and placebo showed significantly increased PFS (11 vs. 5.7 months, HR for progression or death 0.65; 95% CI, 0.50–0.85; P<0.001) in the PIK3CA-mutated cohort of patients with ER-positive/HER-2 negative advanced BC (24). This study demonstrates the utility in selecting isoform-specific PI3K inhibitors although the side effect profile of hyperglycemia and GI upset is still commonly seen in this category.
There is growing evidence that targeting the PI3K pathway in TNBC may be beneficial. The LOTUS trial was a phase II study designed to investigate the efficacy of ipatasertib, an inhibitor of all three AKT isoforms, plus paclitaxel in treatment-naïve locally advanced or metastatic TNBC. It showed PFS was increased with the addition of ipatasertib versus placebo (25). Similarly, the PAKT trial, another phase II trial, found that both median PFS and OS were significantly longer in patients treated with capivasertib plus paclitaxel vs. the placebo plus paclitaxel cohort (26). Although there is much work left to do in considering the treatment for TNBC, these initial studies show promise of the efficacy of PI3K inhibitors in both ER-positive and TNBC.
mTOR inhibitors
mTOR, mammalian target of rapamycin, is a serine-threonine kinase involved in cell growth, differentiation, and autophagy (27). It is involved in the PI3K/AKT pathway, and activating mutations in this pathway have been associated with resistance to cancer treatment, including endocrine therapy (28,29). Everolimus and temsirolimus, analogs of rapamycin, have been studied in clinical trials in BC patients.
The phase III BOLERO-2 trial randomized post-menopausal woman with ER-positive mBC to receive either everolimus plus exemestane (an aromatase inhibitor) or placebo plus exemestane. The addition of everolimus was shown to prolong median PFS (10.6 versus 4.1 months; HR 0.36; 95% CI, 0.27–0.47; P<0.001) (30). In the BOLERO-3 trial, women with HER-2 positive mBC that was resistant to trastuzumab were randomized to receive either everolimus plus trastuzumab plus vinorelbine or placebo plus trastuzumab plus vinorelbine, and median PFS was longer in the group that received everolimus (7 vs. 5.8 months; HR 0.78; 95% CI, 0.65–0.95; P=0.0067) (31). The most common toxicities that occurred with the addition of everolimus were neutropenia, leukopenia, anemia, mucositis, and fatigue. Other trials evaluating the efficacy of everolimus are underway (NCT02313051, NCT01783444).
Her-2 positive BC
Dual anti-HER-2 therapy
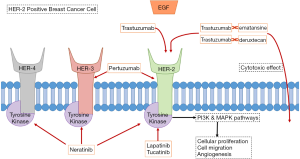
For years, the mainstay of treatment for metastatic HER-2 positive BC has been utilizing trastuzumab, a monoclonal antibody targeting HER-2 (see Figure 1), in combination with chemotherapeutic agents such as paclitaxel or docetaxel (32). As per the CLEOPATRA trial, using dual anti-HER-2 therapy by adding pertuzumab to the combination of trastuzumab and docetaxel in patients with HER-2 positive mBC improved median overall survival (OS) by more than 15 months (HR 0.68; 95% CI, 0.56–0.84; P<0.001). Pertuzumab also extended the median duration of response by 7.7 months (33). Adding pertuzumab increased the risk of mucositis, diarrhea, and rash. Given the results of this trial, HER-2 positive mBC is now routinely being treated with dual anti-HER-2 therapy (trastuzumab and pertuzumab) and a taxane.
Trastuzumab emtansine and trastuzumab deruxtecan
Another important development was the production of trastuzumab emtansine (also known as T-DM1). This is a conjugate of trastuzumab with the drug emtansine (a microtubule inhibitor), combining the anti-HER-2 effect of trastuzumab with cytotoxic effect of emtansine, being delivered in a targeted fashion to cancer cells expressing HER-2 (see Figure 1) (34). The EMILIA phase III trial compared trastuzumab emtansine versus lapatinib plus capecitabine in patients with advanced HER-2 positive BC who had undergone prior treatment with trastuzumab and a taxane (35). Median PFS was 9.6 months with trastuzumab emtansine versus 6.4 months with lapatinib plus capecitabine (HR for progression or death from any cause 0.65; 95% CI, 0.55–0.77; P<0.001). Also, OS was 30.9 months with trastuzumab emtansine versus 25.1 months lapatinib plus capecitabine (HR 0.68; 95% CI, 0.55–0.85; P<0.001), and the objective response rate (ORR) was 43.6% with trastuzumab emtansine versus 30.8% with lapatinib plus capecitabine (P<0.001). The common toxicities associated with trastuzumab emtansine were thrombocytopenia and transaminitis.
The TH3RESA trial was another phase III trial that also recruited patients with HER-2 positive mBC and compared trastuzumab emtansine against the physicians’ choices of treatment. The trial demonstrated comparable improvements in PFS and OS in the trastuzumab emtansine group with lower rates of grade 3 or worse adverse events (36). Based on the results of these trials, trastuzumab emtansine is now recommended in patients with HER-2 positive mBC as second-line therapy or beyond.
Similarly, trastuzumab deruxtecan (DS 8210) combines trastuzumab with a cytotoxic topoisomerase I inhibitor. In a phase II trial published in December 2019, 184 patients with metastatic HER-2 positive BC who had undergone prior treatment with T-DM1 were treated with trastuzumab deruxtecan; 60.9% of patients responded to therapy, with a median PFS of 16.4 months (37). As a result of these findings, the FDA has granted accelerated approval of trastuzumab deruxtecan for metastatic HER-r positive BC following two or more prior anti-HER2 treatments (38).
HER-2 tyrosine kinase inhibitors
Lapatinib and neratinib are HER-2-specific tyrosine kinase inhibitors (TKI) that have shown efficacy in the management of BCs expressing HER-2 (see Figure 1) (39). While lapatinib has been studied in the past and has been approved for use with capecitabine against HER-2 positive BCs that progressed after treatment (40), neratinib has only been more recently studied. So far, neratinib has mostly been shown to be effective in local BC (41), but there are promising results of efficacy from a phase 1b trial of patients with metastatic HER-2 positive BC treated with T-DM1 plus neratinib in patients who progressed on trastuzumab plus pertuzumab (42). In the trial, 63% of patients had a response to treatment. Side effects included diarrhea, thrombocytopenia, and nausea.
In a study published in February 2020, tucatinib, another HER-2 TKI, was compared to placebo in combination with trastuzumab and capecitabine in patients with HER-2 positive BC who had been previously treated with trastuzumab, pertuzumab, and T-DM1 (43). PFS at 1 year was 33.1% in the tucatinib group and 12.3% in the placebo (HR 0.54; 95% CI, 0.42–0.71; P<0.001). Two-year OS was 44.9% vs. 26.6% in the tucatinib group and vs. the placebo group (HR 0.66; 95% CI, 0.50–0.88; P=0.005). Common side effects seen were diarrhea, nausea, vomiting, and palmar-plantar erythrodysesthesia.
TNBC
Immunotherapy
Programmed cell death protein 1 (PD-1) is an immune checkpoint protein on lymphocyte cell surfaces responsible for suppressing regulatory T cell activity, thus reducing apoptosis by guarding against autoimmunity and preventing the immune system from killing cancer cells. Programmed cell death ligand-1 (PD-L1) is a protein expressed on tumor cells which reduces the activity of T cells by binding to PD-1. Checkpoint inhibitors can block inhibitory signals to T cell activation by blocking either PD-L1 (e.g., atezolizumab) or PD-1 (e.g., pembrolizumab), therefore allowing the immune system to recognize and destroy cancer cells (44). TNBC is considered the most likely type of BC to respond to immunotherapy, as it has the greatest mutational frequency, increasing the chance of expressing immunogenic neoantigens (45), and TNBC tumors tend to contain notable amounts of tumor-infiltrating lymphocytes (46). Pembrolizumab was initially studied in the phase 1b Keynote-012 study in 32 women with metastatic TNBC (of which 58.6% had PD-L1-positive disease). It demonstrated acceptable tolerability with an 18.5% overall response rate. Of note, there was an increased probability of response in this trial with increasing PD-L1 expression (47). As of now, pembrolizumab combined with chemotherapy is undergoing a phase III Keynote-355 trial in patients with inoperable or metastatic TNBC (NCT02819518) (48), and a phase III Keynote-119 trial being compared to chemotherapy as a single agent, also for metastatic TNBC (NCT02555657).
The IMpassion 130 trial, a phase III trial published in late 2018, showed significant benefit with atezolizumab was combined with nab-paclitaxel (49). The trial randomly assigned 92 patients with untreated metastatic TNBC to receive atezolizumab plus nab-paclitaxel (AP) or placebo plus nab-paclitaxel (PP). The median PFS was 1.7 months longer in the AP group compared to the PP group (7.2 vs. 5.5 months; HR for progression or death, 0.80; 95% CI, 0.69–0.92; P=0.002). Although the median overall survival was 3.7 months longer in the AP group compared to the PP group, it was not statistically significant (21.3 vs. 17.6 months; HR for death, 0.84; 95% CI, 0.69 to 1.02; P=0.08).
However, in patients with PD-L1 positive tumors (determined by PD-L1 expression on tumor-infiltrating immune cells), the median PFS was 2.5 months longer in the AP group compared to the PP group (7.5 vs. 5.0 months; HR 0.62; 95% CI, 0.49–0.78; P<0.001). The median overall survival in this category of patients was 9.5 months longer in the AP group compared to the PP group and was statistically significant (25.0 vs. 15.5 months; HR for death 0.62; 95% CI, 0.45 to 0.86). The results of the IMpassion 130 trial led to the approval of atezolizumab for PD-L1 positive BC.
Nivolumab (an anti-PD-1 agent) was studied in the TONIC phase II trial to determine if pre-treatment of metastatic TNBC with radiotherapy or chemotherapy (cyclophosphamide, cisplatin or doxorubicin) had any role in improving response to PD-1 blockade (50). Most responses were observed in the doxorubicin group (ORR 35%) and cisplatin group (ORR 23%). The ORR of the entire cohort was 20%. Notably, there was an increased expression of genes involved in the PD-1/PD-L1 pathway after treatment with these two agents. These results suggest there is a role for induction chemotherapy to enhance the immunogenicity of TNBC and improve responses to checkpoint inhibitors.
Recent data from the phase II SAFIR02-IMMUNO trial showed that durvalumab (another anti-PD-L1 agent) was effective as maintenance therapy when compared to chemotherapy in TNBC patients following first or second-line chemotherapy (51). Among 82 patients with TNBC, the median OS was 21 months with maintenance durvalumab compared to 14 months with chemotherapy (HR 0.54; 95% CI, 0.30–0.97; P=0.0377). In patients with PD-L1-positive disease, the median OS was 26 months with durvalumab compared to 12 months with chemotherapy (HR 0.42; 95% CI, 0.17–1.05; P=0.0552).
Patients with germline BRCA mutations—PARP inhibitors
Polyadenosine diphosphate-ribose polymerase (PARP) inhibitors have shown efficacy in patients with metastatic HER2-negative BC and germline BC susceptibility gene (BRCA) mutations. PARP inhibitors work by inducing cell death. In a normal cell cycle repair pathway, PARP 1 and 2 proteins help repair single-stranded breaks (SSB) in DNA. When these breaks go unrepaired, double-stranded breaks (DSB) can form during DNA replication. BRCA1 and BRCA2 (BRCA1/2) are proteins that are involved in DNA repair of DSB by homologous recombinant (HR) repair pathway (52). Notably, when BRCA1 and BRCA2 genes are mutated, cells become deficient in HR repair and DNA is repaired with non-homologous end joining which gives rise to additional DNA alterations and deletions leading to increased cancer risk. PARP inhibitors work by causing persistent SSB in some cases leading to DSB by trapping PARP proteins on DNA in addition to blocking their catalytic activity, thus causing cytotoxic effects in the cell (53).
Olaparib, talazoparib, niraparib, veliparib, and rucaparib are PARP inhibitors of which olaparib and talazoparib are specifically approved for mBC. The phase III, open-label OlympiAD trial randomly assigned 302 patients with germline BRCA mutations and HER2-negative mBC to receive oral olaparib monotherapy or standard chemotherapy per the physicians’ choice (54). These patients had all previously received no more than two chemotherapy regimens for their mBC and those with ER-positive disease had received prior endocrine therapy. The median PFS was 2.8 months longer in the olaparib group compared to in the standard-therapy group (7.0 vs. 4.2 months) and the risk of disease progression or death was 42% lower with olaparib monotherapy than with standard therapy (HR for disease progression or death, 0.58; 95% CI, 0.43–0.80; P<0.001). Additionally, the olaparib group had double the response rate compared to the standard therapy group (59.9% vs. 28.8%). The grade 3 or higher adverse events rate was lower in the olaparib group compared to the standard chemotherapy group (36.6% vs. 50.5%). Toxicities that occurred more frequently in the olaparib group included anemia, vomiting, fatigue, and cough. While the overall survival did not significantly differ between the two treatment groups (HR 0.90; 95% CI, 0.63–1.29; P=0.57), the trial was not powered to assess this difference. Moreover, there were confounding variables such as subsequent treatment in patients specifically those in the standard therapy group which limited the findings.
Similar to the OlympiAD trial, the phase III EMBRACA RCT was done to study talazoparib in patients with advanced BC and germline BRCA mutations who had received appropriate prior chemotherapy and endocrine therapy (55). Four hundred and thirty-one patients were randomized to receive talazoparib monotherapy or standard chemotherapy with a single agent as per the physician’s choice. The median PFS was 3 months longer in the talazoparib group compared to in the standard-therapy group (8.6 vs. 5.6 months) and the risk of disease progression or death was lower with talazoparib monotherapy than with standard therapy (HR for disease progression or death, 0.54; 95% CI, 0.41–0.71; P<0.001). Additionally, the talazoparib group had approximately double the response rate compared to the standard therapy group (62.6% vs. 27.2%). The grade 3 or higher adverse events rate were similar in both, the talazoparib group and the standard chemotherapy group (25.5% vs. 25.4%), but more hematologic adverse events (anemia) occurred in talazoparib vs. standard group (55% vs. 38%) and less non-hematologic adverse events occurred in talazoparib vs. standard group (32% vs. 38%).
Other therapies still under investigation
Androgen receptor antagonists
In a meta-analysis that included more than 2,800 patients with TNBC, nearly a quarter of them were androgen-receptor positive (56), and several trials have demonstrated an increased response of TNBC to androgen antagonists such as bicalutamide and abiraterone acetate (57,58).
HDAC, AKT-1, and FGF receptor inhibitors
Entinostat, a histone deacetylase (HDAC) inhibitor, seems to have shown some benefit when combined with exemestane in a phase 2 trial (59) and is now being studied in a phase 3 trial (NCT02115282). Capivasertib (AZD5363), an AKT-1 inhibitor, is also under investigation. The BEECH study, consisting of both phase 1 and phase 2 trials, showed that capivasertib was well tolerated with paclitaxel in patients with advanced HR-positive BC, but did not produce a significant difference in median PFS (60). Lucitanib, an inhibitor of fibroblast growth factor (FGF) receptor, was found to induce tumor response in about 20–50% of FGF-aberrant BCs in phase I & II trials (61,62).
Promising novel HER-2 targeted agents
Based on data from interim analysis of the phase III SOPHIA trial released in late 2019, margetuximab, an anti-HER-2 monoclonal antibody, plus chemotherapy improved OS in metastatic HER-2 BC patients by 1.8 months (21.6 months) compared with trastuzumab plus chemotherapy (19.8 months) (HR 0.885; 95% CI, 0.693–1.130; P=0.326) (63). The complete analysis is expected to arrive in 2020.
Promising novel options for TNBC
In a recent study by Vaidya et al., nano-particle mediated RNAi were used in-vitro to target long noncoding RNA sequences in TNBC cells (64). The treatment demonstrated efficacy via a significant reduction in the invasion, migration, survival, and proliferation of the TNBC cells that were targeted. Administration in mice who had TNBC xenografts resulted in suppression of TNBC progression and was well tolerated. While it may be some time before this reaches clinical trials, this is a treatment method to keep an eye on.
Sacituzumab govitecan is a combination of an antibody that targets trophoblast cell-surface antigen 2 (Trop-2) with SN-38, an active metabolite of irinotecan. When studied in phase I/II trials of patients with metastatic TNBC who had undergone prior treatments (65,66), sacituzumab govitecan resulted in around a 30% response rate and median response durations of about 8 months. The drug was relatively well tolerated, with the most common adverse events being myelosuppression and diarrhea. It is currently being evaluated in the phase III ASCENT trial (NCT02574455).
Conclusions
Despite the mortality associated with mBC, there have been significant advances made in discovering novel agents to treat this disease (Table 1). New potential options for patients with ER-positive cancers include CDK4/6 inhibitors, mTOR inhibitors, and PI3K inhibitors. Advances in HER-2 positive BC include combining anti-HER-2 therapy, trastuzumab emtansine, trastuzumab deruxtecan (DS 8210), neratinib and tucatinib. Metastatic TNBC can be treated with PARP inhibitors and, given their immunogenicity, respond to anti-PD1/PD-L1 immunotherapy. These agents provide new treatment methods to improve outcomes in patients with mBC, and research is underway to find more options to help us overcome this challenging disease.

Full table
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Khalid Sossey-Alaoui) for the Series “Cancer Metastasis: Molecular signaling and therapeutic options” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.43). The series “Cancer Metastasis: Molecular signaling and therapeutic options” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Facts and Figures 2019. American Cancer Society.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341-52. [Crossref] [PubMed]
- Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast. J Clin Oncol 2013;31:3997-4013. [Crossref] [PubMed]
- Gluz O, Liedtke C, Gottschalk N, et al. Triple-negative breast cancer--current status and future directions. Ann Oncol 2009;20:1913-27. [Crossref] [PubMed]
- Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov 2016;6:353-67. [Crossref] [PubMed]
- Santamaria D, Ortega S. Cyclins and CDKS in development and cancer: lessons from genetically modified mice. Front Biosci 2006;11:1164-88. [Crossref] [PubMed]
- Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009;11:R77. [Crossref] [PubMed]
- Warenius HM, Kilburn JD, Essex JW, et al. Selective anticancer activity of a hexapeptide with sequence homology to a non-kinase domain of Cyclin Dependent Kinase 4. Mol Cancer 2011;10:72. [Crossref] [PubMed]
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25-35. [Crossref] [PubMed]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-36. [Crossref] [PubMed]
- Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015;373:209-19. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738-48. [Crossref] [PubMed]
- Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019;381:307-16. [Crossref] [PubMed]
- Sledge GW, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol 2017;35:2875-84. [Crossref] [PubMed]
- Sledge GW, Toi M, Neven P, et al. The Effect of Abemaciclib plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol 2019. [Epub ahead of print]. [PubMed]
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638-46. [Crossref] [PubMed]
- Yu Y, Savage RE, Eathiraj S, et al. Targeting AKT1-E17K and the PI3K/AKT Pathway with an Allosteric AKT Inhibitor, ARQ 092. PLoS One 2015;10:e0140479. [Crossref] [PubMed]
- Pereira B, Chin SF, Rueda OM, et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun 2016;7:11479. [Crossref] [PubMed]
- Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol 2012;30:282-90. [Crossref] [PubMed]
- Rodon J, Braña I, Siu LL, et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs 2014;32:670-81. [Crossref] [PubMed]
- Baselga J, Im SA, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:904-16. [Crossref] [PubMed]
- Campone M, Im SA, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant for postmenopausal, hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer: Overall survival results from BELLE-2. Eur J Cancer 2018;103:147-54. [Crossref] [PubMed]
- Mayer IA, Abramson VG, Formisano L, et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Ka-Specific Inhibitor, with Letrozole in ERþ/HER2 Metastatic Breast Cancer. Clin Cancer Res 2017;23:26-34. [Crossref] [PubMed]
- André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 2019;380:1929-40. [Crossref] [PubMed]
- Kim SB, Dent R, Im SA, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2017;18:1360-72. [Crossref] [PubMed]
- Schmid P, Abraham J, Chan S, et al. AZD5363 plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (PAKT): A randomised, double-blind, placebo-controlled, phase II trial. J Clin Oncol 2018;36:1007. [Crossref]
- Sulaimanov N, Klose M, Busch H, et al. Understanding the mTOR signaling pathway via mathematical modeling. Wiley Interdiscip Rev Syst Biol Med 2017;9:e1379. [Crossref] [PubMed]
- Burris HA. Overcoming acquired resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR pathway. Cancer Chemother Pharmacol 2013;71:829-42. [Crossref] [PubMed]
- Sabatini DM. mTOR and cancer: Insights into a complex relationship. Nat Rev Cancer 2006;6:729-34. [Crossref] [PubMed]
- Beaver JA, Park BH. The BOLERO-2 trial: The addition of everolimus to exemestane in the treatment of postmenopausal hormone receptor-positive advanced breast cancer. Future Oncol 2012;8:651-7. [Crossref] [PubMed]
- André F, O’Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014;15:580-91. [Crossref] [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724-34. [Crossref] [PubMed]
- Junttila TT, Li G, Parsons K, et al. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011;128:347-56. [Crossref] [PubMed]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- Krop IE, Kim SB, Gonzalez-Martin A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689-99. [Crossref] [PubMed]
- Modi S, Saura C, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 2020;382:610-21. [Crossref] [PubMed]
- FDA approves fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive breast cancer | FDA.
- Xuhong JC, Qi XW, Zhang Y, et al. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am J Cancer Res 2019;9:2103-19. [PubMed]
- Ryan Q, Ibrahim A, Cohen MH, et al. FDA Drug Approval Summary: Lapatinib in Combination with Capecitabine for Previously Treated Metastatic Breast Cancer That Overexpresses HER-2. Oncologist 2008;13:1114-9. [Crossref] [PubMed]
- Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1688-700. [Crossref] [PubMed]
- Abraham J, Montero AJ, Jankowitz RC, et al. Safety and efficacy of T-DM1 plus neratinib in patients with metastatic HER2-positive breast cancer: NSABP Foundation Trial FB-10. J Clin Oncol 2019;37:2601-9. [Crossref] [PubMed]
- Murthy RK, Loi S, Okines A, et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med 2020;382:586. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Budczies J, Bockmayr M, Denkert C, et al. Classical pathology and mutational load of breast cancer - integration of two worlds. J Pathol Clin Res 2015;1:225-38. [Crossref] [PubMed]
- García-Teijido P, Cabal ML, Fernández IP, et al. Tumor-infiltrating lymphocytes in triple negative breast cancer: The future of immune targeting. Clin Med Insights Oncol 2016;10:31-9. [PubMed]
- Nanda R, Chow LQM, Dees EC, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 2016;34:2460-7. [Crossref] [PubMed]
- Cortés J, Guo Z, Karantza V, et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo (PBO) + chemo for previously untreated, locally recurrent, inoperable or metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 2018;36:TPS18. [Crossref]
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018;379:2108-21. [Crossref] [PubMed]
- Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med 2019;25:920-8. [Crossref] [PubMed]
- Dalenc F, Bachelot T, Fllleron T, et al. Durvalumab compared to maintenance chemotherapy in patients with metastatic breast cancer: Results from phase II randomized trial SAFIR02-IMMUNO. Presented at: 2019 San Antonio Breast Cancer Symposium; December 10.
- Chalmers AJ. The potential role and application of PARP inhibitors in cancer treatment. Br Med Bull 2009;89:23-40. [Crossref] [PubMed]
- Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017;355:1152-8. [Crossref] [PubMed]
- Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017;377:523-33. [Crossref] [PubMed]
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018;379:753-63. [Crossref] [PubMed]
- Wang C, Pan B, Zhu H, et al. Prognostic value of androgen receptor in triple negative breast cancer: A meta-analysis. Oncotarget 2016;7:46482-91. [Crossref] [PubMed]
- Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res 2013;19:5505-12. [Crossref] [PubMed]
- Bonnefoi H, Grellety T, Tredan O, et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1). Ann Oncol 2016;27:812-8. [Crossref] [PubMed]
- Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 2013;31:2128-35. [Crossref] [PubMed]
- Turner NC, Alarcón E, Armstrong AC, et al. BEECH: a dose-finding run-in followed by a randomised phase II study assessing the efficacy of AKT inhibitor capivasertib (AZD5363) combined with paclitaxel in patients with estrogen receptor-positive advanced or metastatic breast cancer, and in a PIK3CA mutant sub-population. Ann Oncol 2019;30:774-80. [Crossref] [PubMed]
- Soria JC, DeBraud F, Bahleda R, et al. Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann Oncol 2014;25:2244-51. [Crossref] [PubMed]
- Hui R, Pearson A, Cortés J, et al. Lucitanib for the Treatment of HR+/HER2- Metastatic Breast Cancer: Results from the Multicohort Phase II FINESSE Study. Clin Cancer Res 2020;26:354-63. [Crossref] [PubMed]
- MacroGenics Announces Second Interim Overall Survival Data from Phase 3 SOPHIA Study of Margetuximab in Patients with HER2-Positive Metastatic Breast Cancer | MacroGenics, Inc.
- Vaidya AM, Sun Z, Ayat N, et al. Systemic Delivery of Tumor-Targeting siRNA Nanoparticles against an Oncogenic LncRNA Facilitates Effective Triple-Negative Breast Cancer Therapy. Bioconjug Chem 2019;30:907-19. [Crossref] [PubMed]
- Bardia A, Mayer IA, Diamond JR, et al. Efficacy & safety of anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol 2017;35:2141-8. [Crossref] [PubMed]
- Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med 2019;380:741-51. [Crossref] [PubMed]