Associations of myocardial bridging with adverse cardiac events: a meta-analysis of published observational cohort studies involving 4,556 individuals
Introduction
Myocardial bridging (MB) is a congenital variant of coronary artery anatomy which indicates the myocardium overlying an intramural segment of an epicardial coronary artery. MB mostly involves the middle segment of the left anterior descending artery (LAD), though its prevalence varies according to different imaging modalities and methods used (1-3). The data derived from small sample studies indicate MB may cause a variety of adverse cardiac events (ACEs) including myocardial infarction (MI), life-threatening arrhythmias, and sudden cardiac death (3-6). In this regard, the clinical relevance of MB is of crucial importance. Actually, MB has long been considered as a benign condition given that the prevalence of MB is usually high in autopsy and blood flow runs through normal coronary artery mainly during diastolic phase, while MB compression occurs during systolic phase and only approximately in one third of subjects with MB (3,7-10). Therefore, the precise clinical implication of MB on prognosis remains controversial. We aimed to conduct a meta-analysis of currently available evidence to examine the clinical implication of MB on prognosis among general population.
Methods
The present meta-analysis was performed with a predefined protocol and complied with PRISMA and MOOSE guidelines (Table S1,S4).

Full table
Full table
Full table
Full table
Search strategy
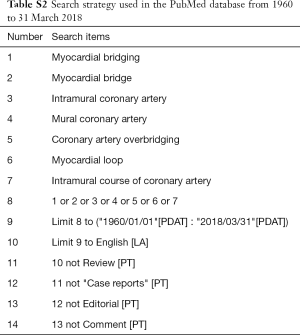
An extensive search of PubMed with English language restriction was performed using the terms like “myocardial bridging”, “myocardial bridge”, “intramural coronary artery”, “mural coronary artery”, “coronary artery overbridging”, “tunneled artery” and “myocardial loop”. Additional reference lists of relevant articles were reviewed. Studies published between 1960 in which year MB was first reported angiographically and 31 March 2018 were identified (3,4,11). The detailed search strategy was presented in Table S2.
Study selection
We only included observational cohort studies either prospective or retrospective comparing the outcome of subjects with and without MB, which represent the best level of clinical evidence to date. Inclusion criteria were the followings: (I) population referred consecutively to hospital for imaging examination of coronary artery; (II) explicit description of inclusion or exclusion criteria; and (III) comparison of outcome during follow-up between subjects with and without MB. Exclusion criteria were the followings: (I) studies incapable of extracting specific data; (II) studies dealing with patient population with specific disease like hypertrophic cardiomyopathy. Potentially eligible studies were evaluated by two independent reviewers (C Zhu and S Wang) as well as data extraction and quality evaluation of the final included studies. Any discrepancies were resolved by consensus meeting of all authors of this meta-analysis subsequently.
Quality evaluation of included studies
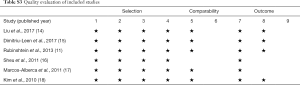
The Newcastle-Ottawa Scale for cohort studies, which is a “star system” providing an easy and convenient quality assessment of nonrandomized studies in a systematic review, was used to evaluate the quality of included studies on three perspectives: selection of cohorts, comparability of cohorts, and ascertainment of outcome for cohorts (12,13). Nine stars represent the highest study quality. At least 5 stars were defined to be adequate quality for inclusion in the present meta-analysis. With regard to evaluation for publication bias of included studies, the visualized funnel plot was used if applicable.
Outcomes
The primary outcome was defined as ACEs including cardiovascular death and non-fatal MI. Secondary outcomes were non-fatal MI, angina requiring hospitalization, and all-cause mortality. Furthermore, a composite endpoint was defined as a combination of ACEs, non-cardiac death and angina requiring hospitalization.
Statistical analysis
Analysis was conducted using Review Manager Version 5.3 (The Cochrane Collaboration, Update Software, Copenhagen, The Nordic Cochrane Centre). Heterogeneity test was measured utilizing the v2 test (Cochrane’s Q) and I2 value. I2 values less than 50%, 50% to 75%, and more than 75% represent a low, moderate, high degree of heterogeneity, respectively. If homogenous, fixed-effect model was used. Otherwise, a random-effects model was used. Odds ratio (OR) was calculated for dichotomous variables with 95% confidence interval (CI). An OR represents the ratio between odds of outcomes in the context of a particular exposure and odds of outcomes in absence of the exposure. A P value of less than 0.05 was considered statistically significant.
Results
Selection of studies
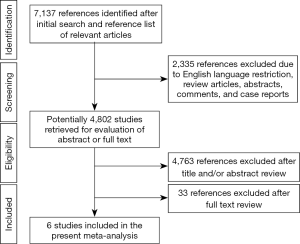
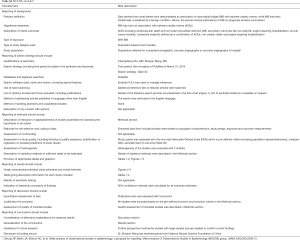
Six observational cohort studies were included in the present meta-analysis for data extraction which yielded a total of 4,556 selected subjects (Figure 1) (11,14-18). Of these six included studies, only the study by Rubinshtein et al. was prospective, whereas the remaining 5 studies were retrospective. The study by Rubinshtein et al. included subjects with compromised left ventricular function or valvular heart disease who were referred to rule out obstructive coronary artery disease (11). In contrast, the study by Kim et al. excluded subjects with any risk factors of chest pain including valvular heart disease (18). The detailed inclusion and exclusion criteria and outcome measurements of selected studies were presented in Table 1. Besides, the study by Kim et al. assessed MB with coronary angiography. Table 2 demonstrates data extracted from all included studies in the present meta-analysis. All subjects were in absence of prior coronary heart disease or obstructive coronary artery disease which was defined as equal to or more than 50% coronary luminal stenosis of any coronary artery.
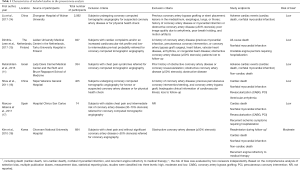
Full table
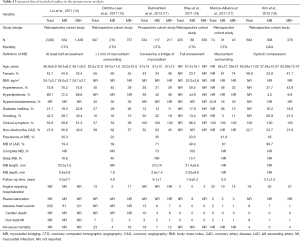
Full table
The quality evaluation of selected studies was demonstrated in Table S3. None of these six included studies provided information on losses to follow-up.
Pooled prevalence and characteristics of MB
Of the 4,556 selected subjects included, 1,389 had MB. Thus, the pooled prevalence of MB in the present study is 30.5%. Most MB involved the LAD, which was consistent among included studies. Three studies reported MB with mean length of 2 to 3 mm and mean depth of 2.6 mm (11,14,16).
Primary outcome
ACEs were reported in five included studies comprising a total of 225 events among 3,609 subjects (11,14,16-18). The pooled incidences of ACEs were 8.1% and 5.3% in subjects with MB and without MB, respectively. On pooled analysis, subjects with MB had an increased risk of ACEs compared with subjects without MB (OR: 1.71; 95% CI: 1.29 to 2.26, P=0.0002) (Figure 2A). There was no statistical significance of heterogeneity test between included studies (Cochrane Q =2.46, P=0.48, I2 =0%). Besides, the corresponding funnel plot indicated that no publication bias existed (Figure 2B).

Sensitivity analysis was performed by only including five studies which used coronary computed tomographic angiography for detection of MB. Results were unchanged for ACEs in subjects with MB compared to that in subjects without MB (OR: 1.62; 95% CI: 1.21 to 2.15, P=0.001) (Figure S1).
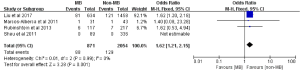
Secondary outcomes
Non-fatal MI was reported in five studies comprising a total of 20 events among 2,464 subjects (11,15-18). The pooled incidences of non-fatal MI were 1.6% and 0.5% in subjects with MB and without MB, respectively. Subjects with MB had an increased risk of experiencing non-fatal MI compared with subjects without MB (OR: 3.17; 95% CI: 1.21 to 8.31, P=0.02) (Figure 3). There was no statistical heterogeneity for the outcome of non-fatal MI between included studies (Cochrane Q =2.17, P=0.54, I2 =0%).

Angina requiring hospitalization was reported in 4 studies comprising a total of 128 events among 2,130 subjects (15-18). The pooled incidences of angina requiring hospitalization were 11.4% and 3.7% in subjects with MB and without MB, respectively. Subjects with MB had an increased risk of angina requiring hospitalization compared with subjects without MB (OR: 2.31; 95% CI: 1.55 to 3.45, P<0.0001) (Figure 4). There was no statistical heterogeneity between included studies (Cochrane Q =4.50, P=0.21, I2 =33%).

All-cause mortality was reported in three studies comprising a total of 46 events among 1,965 subjects (11,15,18). The pooled incidences of all-cause mortality were 1.7% and 2.6% in subjects with MB and without MB, respectively. Subjects with MB had no significant increase in the risk of all-cause mortality compared with subjects without MB (OR: 0.75; 95% CI: 0.38 to 1.49, P=0.41) (Figure S2). There was no statistical heterogeneity between included studies (Cochrane Q =1.90, P=0.39, I2 =0%).
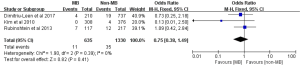
Composite endpoint
Of six included studies, three studies involving 1,183 subjects, reported composite endpoint comprising of ACEs, non-cardiac death and angina requiring hospitalization (16-18). The pooled incidences of the composite endpoint were 18.5% and 6.1% in subjects with MB and without MB, respectively. Subjects with MB had an increased risk of experiencing the composite endpoint compared with subjects without MB (OR: 2.89; 95% CI: 1.90 to 4.39, P<0.00001) (Figure 5). There was no statistical heterogeneity between included studies (Cochrane Q =1.36, P=0.51, I2 =0%).

Discussion
The present meta-analysis aims to examine the impact of MB on clinical prognosis in the general population which includes the latest cohort studies to date as far as we know. Our results indicate that MB is associated with an increased risk of ACEs and non-fatal MI in the present study. Thus, our findings may have important implications with regard to clinical practice and may alter our previous conceptions and strategies to provide more attention and optimal management of MB.
The pooled prevalence of MB with 30.5% in the present study is similar to that in the prospective study by Rubinshtein et al. and the average prevalence of 25% detected in autopsy which is usually regarded as a reference standard (7,11,16). Generally, according to previous studies, depiction rate of MB in coronary angiography, coronary computed tomographic angiography and autopsy is increased in ascending order (4,5,7,11). The prevalence of MB on coronary computed tomographic angiography in more recent studies is in accordance with autopsy series, which may be attributed to the increasingly high spatial resolution of newer generation computed tomography capable of refining MB (4,19).
Our key findings suggest that MB confers an increased risk of ACEs (OR: 1.71; 95% CI: 1.29 to 2.26, P=0.0002) and non-fatal MI (OR: 3.17; 95% CI: 1.21 to 8.31, P=0.02) in subjects with MB compared with subjects without MB, respectively. Thus, our findings are contrary to previous studies and traditional consideration that MB is a normal variant or a benign coronary anomaly (11,15). Regarding clinical symptom, subjects with MB had an increased risk of angina requiring hospitalization (OR: 2.31; 95% CI: 1.55 to 3.45, P<0.0001) compared with subjects without MB.
There are several potential mechanisms that may attribute to the association of MB with ACEs or myocardial ischemia. First, MB itself mostly involves the LAD which is one of the most important coronary arteries and whose lesion commonly contributes to most MI or myocardial ischemia in obstructive coronary artery disease. Hemodynamic relevance of MB differs significantly with regard to its anatomy especially depth (1,20). Second, multiple studies on MB using intracoronary ultrasound, Doppler and quantitative coronary angiography have revealed that systolic compression of MB persists into diastolic phase of cardiac cycle rather than that MB is just a systolic event (21-25). This finding is deemed highly unique as it can only be detected in the segment of MB with systolic compression (1,26). Moreover, findings by intracoronary Doppler demonstrate that MB compression delays luminal recovery in early diastole which may impair diastolic hemodynamics, which is left unidentified before (21). Additionally, the degree of the systolic compression of MB is positively associated with reduction of luminal diameter and corresponding decrease in flow and flow reserve during diastole (27). Third, previous studies reveal endothelial dysfunction of the tunneled coronary artery beneath MB (28,29). Furthermore, reduced expression of some vasoactive agents like endothelial nitric oxide synthase, endothelin-1, and angiotensin-converting enzyme at the MB site were ascertained to attribute to endothelial dysfunction of the tunneled coronary artery, which may predispose tunneled coronary artery to spasm at the same time (4,28,29). Fourth, several studies demonstrated a higher incidence of cardiac death and nonfatal MI in subsets of patients with coronary artery spasm and without obstructive coronary artery disease (30,31). Fifth, it has been found that vessel segment proximal to MB predisposes to development of atherosclerosis or formation of plaques, though vessel segment within MB is protected from development of atherosclerotic lesions (6,10,32). Disturbed retrograde flow produced by systolic compression of MB alters significantly shear stress on the coronary artery wall proximal to MB leading to atherosclerosis of corresponding part of the coronary artery (6,32). This finding has been thought to increase the risk of ACEs or myocardial ischemia.
Limitations
This meta-analysis has some limitations. First, our study itself is prone to inherent limitations of this kind of analysis like publication bias. Our data are confined to rely on published studies. Second, most included studies are retrospective except the study by Rubinshtein and colleagues (11). Therefore, our study may have limitations, potential confounding and biases of all retrospective studies. However, prospectively observational study examining the impact of MB on prognosis is relatively lacking, especially with a comparison group of subjects without MB, and has relatively small sample size. Third, of six included studies, the study by Kim et al. uses coronary angiography to detect MB, which differs from the other five included studies with coronary computed tomographic angiography used and is usually thought to has a lower detection rate of MB. However, Kim et al. administered intracoronary nitroglycerin in order to well define MB once suspecting MB during coronary angiography (18). Besides, six included studies only provided limited information about functional effects of MB or clinical symptoms in participants. Fourth, tools employed for diagnosis of MB may be different among included studies in different periods. However, the time span is relatively short, so differences in terms of anatomical definition and functional relapse are slight. Fifth, follow-up duration in two included studies was relatively short and none of included studies provided information on loss to follow-up, which may add some bias to our study (16,17). Sixth, our study could not respectively refine association of different MB types with presence/magnitude of coronary mal-perfusion and prognosis basing on current evidences due to a lack of source data.
Conclusions
MB is not uncommon especially assessed on coronary computed tomographic angiography. Subjects with MB and without obstructive coronary artery disease have increased risk of experiencing ACEs including cardiac death and non-fatal MI, as well as angina requiring hospitalization. These findings may have substantially important implication which may alter our traditional conception of MB as well as clinical practice. However, the present finding needs further prospectively longitudinal multicenter study with large sample size to validate.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (No. 81570276) and Beijing Science and Technology Program of China (No. Z161100000516154).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tarantini G, Migliore F, Cademartiri F, et al. Left anterior descending artery myocardial bridging: A clinical approach. J Am Coll Cardiol 2016;68:2887-99. [Crossref] [PubMed]
- Corban MT, Hung OY, Eshtehardi P, et al. Myocardial bridging: Contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol 2014;63:2346-55. [Crossref] [PubMed]
- Alegria JR, Herrmann J, Holmes DR Jr, et al. Myocardial bridging. Eur Heart J 2005;26:1159-68. [Crossref] [PubMed]
- Lee MS, Chen CH. Myocardial Bridging: An Up-to-Date Review. J Invasive Cardiol 2015;27:521-8. [PubMed]
- Rogers IS, Tremmel JA, Schnittger I. Myocardial bridges: Overview of diagnosis and management. Congenit Heart Dis 2017;12:619-23. [Crossref] [PubMed]
- Ishikawa Y, Akasaka Y, Suzuki K, et al. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation 2009;120:376-83. [Crossref] [PubMed]
- Möhlenkamp S. Update on myocardial bridging. Circulation 2002;106:2616-22. [Crossref] [PubMed]
- Rossi L, Dander B, Nidasio GP, et al. Myocardial bridges and ischemic heart disease. Eur Heart J 1980;1:239-45. [Crossref] [PubMed]
- Geiringer E. The mural coronary. Am Heart J 1951;41:359-68. [Crossref] [PubMed]
- Uusitalo V, Saraste A, Pietilä M, et al. The Functional Effects of Intramural Course of Coronary Arteries and its Relation to Coronary Atherosclerosis. JACC Cardiovasc Imaging 2015;8:697-704. [Crossref] [PubMed]
- Rubinshtein R, Gaspar T, Lewis BS, et al. Long-term prognosis and outcome in patients with a chest pain syndrome and myocardial bridging: a 64-slice coronary computed tomography angiography study. Eur Heart J Cardiovasc Imaging 2013;14:579-85. [Crossref] [PubMed]
- Wells GA, Shea B, O'Connell O, et al. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses (25 january 2017). 2017. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Kunutsor SK, Seidu S, Khunti K. Statins and secondary prevention of venous thromboembolism: Pooled analysis of published observational cohort studies. Eur Heart J 2017;38:1608-12. [Crossref] [PubMed]
- Liu G, Qu Y, Chen X, et al. Measurements of myocardial bridges on computed tomography predict presence of clinical symptoms and outcomes of adverse heart events: A retrospective study in a large population from china. Acta Radiol 2017;58:1068-76. [Crossref] [PubMed]
- Dimitriu-Leen AC, van Rosendael AR, Smit JM, et al. Long-Term Prognosis of Patients With Intramural Course of Coronary Arteries Assessed With CT Angiography. JACC Cardiovasc Imaging 2017;10:1451-8. [Crossref] [PubMed]
- Sheu MH, Chen YD, Kuo YS, et al. Myocardial bridging in taiwanese: Noninvasive assessment by 64-detector row coronary computed tomographic angiography. J Chin Med Assoc 2011;74:164-8. [Crossref] [PubMed]
- Marcos-Alberca P, Goncalves A, Golfin CF, et al. Clinical outcomes of patients with intramyocardial bridging diagnosed by multi-detector cardiac computed tomography. Int J Cardiol 2011;148:123-5. [Crossref] [PubMed]
- Kim SS, Jeong MH, Kim HK, et al. Long-term clinical course of patients with isolated myocardial bridge. Circ J 2010;74:538-43. [Crossref] [PubMed]
- Konen E, Goitein O, Sternik L, et al. The prevalence and anatomical patterns of intramuscular coronary arteries: A coronary computed tomography angiographic study. J Am Coll Cardiol 2007;49:587-93. [Crossref] [PubMed]
- Leschka S, Koepfli P, Husmann L, et al. Myocardial bridging: Depiction rate and morphology at ct coronary angiography--comparison with conventional coronary angiography. Radiology 2008;246:754-62. [Crossref] [PubMed]
- Escaned J, Cortés J, Flores A, et al. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J Am Coll Cardiol 2003;42:226-33. [Crossref] [PubMed]
- Ge J, Jeremias A, Rupp A, et al. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and doppler. Eur Heart J 1999;20:1707-16. [Crossref] [PubMed]
- Tio RA, Van Gelder IC, Boonstra PW, et al. Myocardial bridging in a survivor of sudden cardiac near-death: role of intracoronary doppler flow measurements and angiography during dobutamine stress in the clinical evaluation. Heart 1997;77:280-2. [Crossref] [PubMed]
- Klues HG, Schwarz ER, vom Dahl J, et al. Disturbed intracoronary hemodynamics in myocardial bridging: Early normalization by intracoronary stent placement. Circulation 1997;96:2905-13. [Crossref] [PubMed]
- Aleksandric S, Djordjevic-Dikic A, Beleslin B, et al. Noninvasive assessment of myocardial bridging by coronary flow velocity reserve with transthoracic doppler echocardiography: Vasodilator vs. Inotropic stimulation. Int J Cardiol 2016;225:37-45. [Crossref] [PubMed]
- Ge J, Erbel R, Rupprecht HJ, et al. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation 1994;89:1725-32. [Crossref] [PubMed]
- Schwarz ER, Klues HG, vom Dahl J, et al. Functional characteristics of myocardial bridging. A combined angiographic and intracoronary doppler flow study. Eur Heart J 1997;18:434-42. [Crossref] [PubMed]
- Kim JW, Seo HS, Na JO, et al. Myocardial bridging is related to endothelial dysfunction but not to plaque as assessed by intracoronary ultrasound. Heart 2008;94:765-9. [Crossref] [PubMed]
- Herrmann J, Higano ST, Lenon RJ, et al. Myocardial bridging is associated with alteration in coronary vasoreactivity. Eur Heart J 2004;25:2134-42. [Crossref] [PubMed]
- Wang CH, Kuo LT, Hung MJ, et al. Coronary vasospasm as a possible cause of elevated cardiac troponin I in patients with acute coronary syndrome and insignificant coronary artery disease. Am Heart J 2002;144:275-81. [Crossref] [PubMed]
- Bory M, Pierron F, Panagides D, et al. Coronary artery spasm in patients with normal or near normal coronary arteries. Long-term follow-up of 277 patients. Eur Heart J 1996;17:1015-21. [Crossref] [PubMed]
- Ishikawa Y, Akasaka Y, Ito K, et al. Significance of anatomical properties of myocardial bridge on atherosclerosis evolution in the left anterior descending coronary artery. Atherosclerosis 2006;186:380-9. [Crossref] [PubMed]