Application of double circular suturing technique (DCST) in the repair of large abdominal wall defects after resection of abdominal wall tumor
Introduction
Large abdominal wall defects may result from abdominal wall trauma, infection, surgery, and other complications, especially in abdominal wall malignancy. As the current mainstream method of surgical treatment is extended resection, large, postoperative, abdominal wall defects have become a significant challenge for surgeons to repair and effectively reconstruct the large abdominal wall (1,2). Fortunately, as the development of biomaterial and tissue transplantation technology has advanced, the repair and reconstruction of the morphology and function of the large abdominal defects have progressed accordingly. Via the interdisciplinarity of general surgery and orthopedic surgery, it is not only possible to recover the appearance and integrity of the abdominal wall, but mechanical support of sufficient strength can be provided for the abdominal wall to guarantee the effect of abdominal wall defect repair (3,4).
Despite these benefits, there remain several problems including high recurrence, complicated operation, many complications, and high costs (5-9). Presently, there were many methods for large abdominal wall defects repair after tumor resection, which included direct suture repair, material implanting repair (synthetic mesh and biological mesh), self-tissue repair, the component separation technique (CST), and so on. However, a simpler and more effective repair method with less complications and low recurrence rate is badly in need. From October 2010 to November 2018, we operated on 62 patients who underwent double circular suturing technique (DCST) in repair of large abdominal wall defects by antiadhesion underlay mesh after resection of abdominal wall tumor. The operations proved to be effective, and a detailed report follows.
Methods
Patient selection
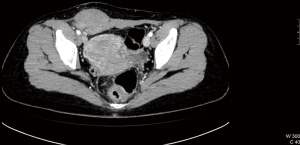
There were 25 men and 37 women whose average age was 41.7±22.4 years. The maximum diameter of abdominal wall defect after resection of abdominal wall tumor was 10.4±5.6 cm. The course of disease was 1–341 months, with an average of 32.4 months. The patients were proven to have abdominal wall tumor preoperatively by means of pathological and imageological examination (computed tomography, ultrasound, magnetic resonance imaging etc.), with no sign of metastasis in the abdominal cavity (example in Figure 1). No patients had intestinal obstruction symptoms and all had undergone selective operations. According to the anesthesia classification system suggested by the American Society of Anesthesiologists (ASA), the patient distribution across levels was as follows: Level I 6 patients; Level II 31 patients; Level III 24 patients; Level IV 1 patient. The retrospective study was approved by the institutional research ethics committee of West China Hospital of Sichuan University.
Operative methods
The repair materials were Proceed® surgical mesh (Ethicon Inc., Johnson & Johnson, USA) or double-deck complex Kugel® mesh (Bard Inc., USA). Both are antiadhesion mesh types which can contact the intestinal canal in the abdominal cavity. The size of the mesh was determined by the size of the defect. The mesh would exceed the edge of the defect by 5 cm in principle, the edge of which could be trimmed properly.
For cases in which a patient was preoperatively and intraoperatively diagnosed as benign tumor, the tumor and its capsule would be resected completely. But when the tumors were suspected as borderline or malignant, the incisal margins should exceed the normal tissue by 2–3 cm to make sure there was no residual tumor on the edge of incisal margins by rapid intraoperative pathological examination.
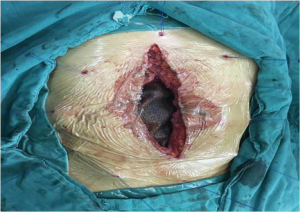
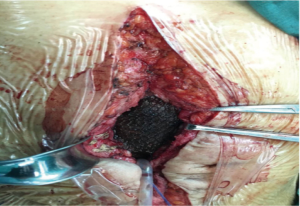
The DCST was performed according to the following steps. First, we fixed the mesh in advance by letting the Prolene suture go through the edge of the mesh discontinuously with an interval of 3–4 cm (Figure 2). Second, we fixed the outer ring by drawing the suture out of the abdominal wall with a perforated puncture needle. It should be noted that entry point of the needle was in the same position under the skin while the needle drilled through the muscular tissue in the different positions; the knot was subcutaneous, and there was firm muscular tissue between the two sutures (Figure 3). We then fixed the inner ring by fixing the mesh with the edge of the ring using non-absorbable or slowly absorbable swaged-on; a knot was tied end to end after a circle of continuous suturing, and care was taken to make a thin puncture while stitching the mesh to avoid bowel injury; we also tried to keep close to the center while suturing to make the orifice smaller after tightening the suture so as to decrease the abdominal closure tension (Figure 4). Finally, we placed the closed drainage and suture and closed the subcutaneous section and the skin. The pathological specimen of the patient was identified as soft tissue desmoid type fibromatosis (Figure 5).
Therapeutic effect assessment index
Operative time, postoperative hospitalization time, perioperative complications, tumor recurrence in situ, incidence of postoperative chronic pain, and hernia were recorded. Visual Analogue Scale (VAS) was used to evaluate the incidence of chronic pain of the incision postoperatively during follow-up; e.g., 0 mm indicated no pain, while 100 mm indicated intolerable pain. The incidence of the tumor recurrence and hernia were evaluated by computed tomography (CT) scanning.
Results
All the 62 operations were completed successfully, and no perioperative deaths occurred. The operative time was 73.2±31.4 minutes and the mean postoperative hospitalization time was 9.6 days (range, 2–20 days). Postoperative pathology showed that there were 11 cases of benign tumor (fobroma and neurofibroma), 5 cases of borderline tumor (desmoid tumor and dermatofibrosarcoma protuberans), 8 cases of primary malignant tumor (fibrosarcoma and liposarcoma), 31 cases of abdominal wall metastatic tumor, and 7 cases of endometriosis. A total of 54 patients (87.1%) were followed up postoperatively for a median of 6.7 years (range, 0.9–9.0 years). Partial incision splitting occurred in 2 patients who were healed by compression bandaging with abdominal bandage and nutritional support treatment; fat liquefaction of incisions occurred in 3 patients who were healed after dressing change; chronic pains occurred in 4 patients, and they were active pain, with the pain score being 2, 3, 3, and 2. All the patients received CT scanning when re-examined, and tumor in situ recurrence, hernia, and other complications did not occur. Tumor metastasis occurred in 9 patients (6 metastatic tumors, 2 fibrosarcomas, and 1 dermatofibrosarcoma protuberans) with 6 of these patients dying of tumour progression.
Discussion
Abdominal wall tumor is a common clinical occurrence. Primary tumor can be formed on the skin or in its appendant organ of the abdominal wall, in the subcutaneous tissue, in the muscle, on the fascia, on the peritoneum, and in other areas. The malignant tumor formed in other parts metastasize to the abdominal wall. The nature of the tumor can be divided into benign, borderline, and malignant types. Lipoma, fibroma, and hemangioma are the most common benign tumors (10). The biological behavior of borderline tumors is between benign and malignant, characterized by relatively slow and invasive growth and without complete development. Abdominal wall malignancy is mostly secondary and results from the invasion and metastasis of intraperitoneal malignancy. Primary abdominal malignancy is usually a soft tissue sarcoma originating from mesenchymal tissue, and can include malignant fibrohistiocytoma, fibrosarcoma, liposarcoma, synovial sarcoma, leiomyosarcoma and other types (11). At present, abdominal wall tumors are mainly treated by surgery, but the recurrence rate of borderline and malignant tumors is high. Of the patients admitted to our center, the proportion of the borderline [5], primary malignant [8] and metastatic malignant [31] abdominal tumor was as high as 70.97% (44/62), which inevitably referred to a large area of the abdominal wall defect after large abdominal wall tumor resection. How to repair abdominal wall defect while assuring the radical cure of the tumor is thus a considerable problem for clinical treatment.
The accurate classification of abdominal wall defect before repair and reconstruction is the foundation of the appropriate surgical procedure selection, and also the premise for evaluating and judging of therapeutic effect. According to the literature, abdominal wall defect can be divided into three types (12): type I, the defect only interferes with the skin and part of the subcutaneous defect; type II, the defect has complete abdominal wall skin and absence of myofascial tissue; type III, the defect is a full-thickness abdominal wall defect. The cases reported belonged to the type II and III defects, and direct suture repair was not recommended. At present, material implanting repair (synthetic mesh and biological mesh), self-tissue repair (CST and autologous musculocutaneous flap), or combined application are recommended.
In 1990, Ramirez et al. were first to report tension-free repair and reconstruction of the abdominal wall defect by means of CST without the use of mesh. Unilateral CST can achieve 5, 10, and 3 cm defect coverage in the upper abdominal wall, waist, and suprapubic region, respectively. Bilateral CST can cover abdominal wall defects of up to 20 cm (13). However, Sailes et al. repaired 545 cases of large and recrudescent abdominal wall hernia by means of CST, and their 10-year retrospective research found that the recurrence rate of abdominal wall hernia reached 18.3% (14). In order to reduce the occurrence of postoperative abdominal wall hernia, CST combined with mesh technique can also be used for patients with large defects or high risk of postoperative recurrence. However, this operation method is intricate and can lead to many complications. Furthermore, CST can only be used in the repair and reconstruction of the region close to the midline, which limits its application (15). The tissue flap technique is more suitable method to repair and reconstruct type III abdominal wall defect; however, the tissue taken from the donor will not only cause the injury and deformity of the donor area, but also be restricted by the supply. It is also entails many complications such as ischemia of the skin flap and hernia. Meanwhile, artificial material implanting involves none of these disadvantages, and as a result, it is better than self-tissue implanting for both specialized situations and general use (16).
Material implanting can meet the demands of tension-free repair of abdominal wall defect. It is widely used in the repair of abdominal wall defect because of its convenience and the fact that it inflicts no new damage to its own tissue. Since the implantation of degradable materials or biological meshes cannot stimulate enough fibrous tissue proliferation, and has a short strength maintenance time and relatively high recurrence rate of hernia, it has been basically rejected in clinical application, with the intensive repair becoming its main use (17). As for the use of non-absorbable materials, onlay and inlay are rarely used in clinical application due to their high postoperative recurrence rate. At present, sublay and underlay are used to recover and reconstruct abdominal wall defect (18). As for the type II abdominal wall defect, after tumor resection combined with peritoneal absence, attention should be paid to the direct connection of mesh and intraperitoneal organs when choosing the mesh. Anti-adhesion complex mesh is used for this kind of repair to avoid the complications like as intestinal adhesion and intestinal fistula. In conclusion, non-absorbable anti-adhesion mesh and sublay are used to repair the large abdominal wall defect after tumor resection and in theory, they accord with the purpose of covering and protecting the organs in the abdominal cavity, reconstructing the appearance of the abdominal wall, and providing the mechanical support with sufficient strength to recover the utmost function and integrity of the abdominal wall.
All the 62 operations were completed successfully with no perioperative deaths or severe complications. Double in situ fixation DCST, and not the type practiced with a wide range of dissociation and release, is simple in operation compared with CST and tissue flap implantation. The operation time was thus only 73.2±31.4 minutes which is much shorter than that of CST and tissue flap implantation (19-21). The decrease of surgical area in the operation can reduce the incidence of the related complications (bleeding, skin necrosis etc.), and no such complications occurred in our patients. In addition, the incidence of postoperative chronic pain was 7.4% (4/54), and all patients had mild pain when moving (VAS score 2–3). DCST not only makes the repair effect much more accurate compared with conventional suspending fixation and margin suture fixation, but also further shrinks the defect to satisfy the principle of low tension when repairing the defect so as to reduce the incidence of postoperative incision split and hernia. In the study, partial incision splitting occurred in only 2 patients who recovered after conservative treatment, and there was no incidence of hernia or tumor in situ recurrence. This demonstrates that the method does not only ensure a sufficient resection range, but also solves the problem of closing a large abdominal wall defect.
Although DCST has several advantages in repairing large defects after abdominal wall tumor resection, its application has some definite limitations. First, for those patients whose bowel is invaded by tumor and who have local severe pollution, non-absorbable material repair is not recommended (22,23). Second, for the patients with large skin defects after abdominal wall tumor resection, self-tissue implanting combined with material repair is necessary.
In conclusion, DCST for the repair of large abdominal wall defects is effective after resection of abdominal wall tumor, and can simplify the operation procedure, shorten the operation time, reduce postoperative complications, and lower the incidence of tumor recurrence, hernia, and postoperative chronic pain.
Acknowledgments
Funding: The National Natural Science Foundation of China (Grant No. 71804117).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional research ethics committee of West China Hospital of Sichuan University. Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Novitsky YW, Elliott HL, Orenstein SB, et al. Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. The Am J Surg 2012;204:709-16. [Crossref] [PubMed]
- Köckerling F, Alam NN, Antoniou SA, et al. What is the evidence for the use of biologic or biosynthetic meshes in abdominal wall reconstruction? Hernia 2018;22:249-69. [Crossref] [PubMed]
- Patel NG, Ratanshi I, Buchel EW. The Best of Abdominal Wall Reconstruction. Plast Reconstr Surg 2018;141:113e-36e. [Crossref] [PubMed]
- Yang F. Radical tumor excision and immediate abdominal wall reconstruction in patients with aggressive neoplasm compromised full-thickness lower abdominal wall. Am J Surg 2013;205:15-21. [Crossref] [PubMed]
- Kuwahara H, Salo J, Tukiainen E. Diaphragm reconstruction combined with thoraco-abdominal wall reconstruction after tumor resection. J Plast Surg Hand Surg 2018;52:172-7. [Crossref] [PubMed]
- Pearson DG, Carbonell AM. Obesity and Abdominal Wall Reconstruction. Plast Reconstr Surg 2018;142:30S-5S. [Crossref] [PubMed]
- Parker M, Bray JM, Pfluke JM, et al. Preliminary experience and development of an algorithm for the optimal use of the laparoscopic component separation technique for myofascial advancement during ventral incisional hernia repair. J Laparoendosc Adv Surg Tech A 2011;21:405-10. [Crossref] [PubMed]
- Wang CM, Zhang R, Luo P, et al. Reconstruction of extensive thoracic wall defect using the external oblique myocutaneous flap: An analysis on 20 Chinese patients with locally advanced soft tissue sarcoma. J Surg Oncol 2018;117:130-6. [Crossref] [PubMed]
- Mathes SJ, Steinwald PM, Foster RD, et al. Complex abdominal wall reconstruction: a comparison of flap and mesh closure. Ann Surg 2000;232:586-96. [Crossref] [PubMed]
- Stojadinovic A, Hoos A, Karpoff HM, et al. Soft tissue tumors of the abdominal wall: analysis of disease patterns and treatment. Arch Surg 2001;136:70-9. [Crossref] [PubMed]
- Smith HG, Tzanis D, Messiou C, et al. The management of soft tissue tumours of the abdominal wall. Eur J Surg Oncol 2017;43:1647-55. [Crossref] [PubMed]
- Khansa I, Janis JE. Modern reconstructive techniques for abdominal wall defects after oncologic resection. J Surg Oncol 2015;111:587-98. [Crossref] [PubMed]
- Ramirez OM, Ruas E, Dellon AL. "Components separation" method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg 1990;86:519-26. [Crossref] [PubMed]
- Sailes FC, Walls J, Guelig D, et al. Synthetic and biological mesh in component separation: a 10-year single institution review. Ann Plast Surg 2010;64:696-8. [PubMed]
- Clarke JM. Incisional hernia repair by fascial component separation: results in 128 cases and evolution of technique. Am J Surg 2010;200:2-8. [Crossref] [PubMed]
- Roubaud MS, Baumann DP. Flap Reconstruction of the Abdominal Wall. Semin Plast Surg 2018;32:133-40. [Crossref] [PubMed]
- Yezhelyev MV, Deigni O, Losken A. Management of full-thickness abdominal wall defects following tumor resection. Ann Plast Surg 2012;69:186-91. [Crossref] [PubMed]
- Deerenberg EB, Timmermans L, Hogerzeil DP, et al. A systematic review of the surgical treatment of large incisional hernia. Hernia 2015;19:89-101. [Crossref] [PubMed]
- de Vries Reilingh TS, van Goor H, Charbon JA, et al. Repair of giant midline abdominal wall hernias: “components separation technique” versus prosthetic repair. World J Surg 2007;31:756-63. [Crossref] [PubMed]
- Wong CH, Lin CH, Fu B, et al. Reconstruction of complex abdominal wall defects with free flaps: indications and clinical outcome. Plast Reconstr Surg 2009;124:500-9. [Crossref] [PubMed]
- Koshima I, Nanba Y, Tutsui T, et al. Dynamic reconstruction of large abdominal defects using a free rectus femoris musculocutaneous flap with normal motor function. Ann Plast Surg 2003;50:420-4. [Crossref] [PubMed]
- Hodgkinson JD, Maeda Y, Leo CA, et al. Complex abdominal wall reconstruction in the setting of active infection and contamination: a systematic review of hernia and fistula recurrence rates. Colorectal Dis 2017;19:319-30. [Crossref] [PubMed]
- Kanters AE, Krpata DM, Blatnik JA, et al. Modified hernia grading scale to stratify surgical site occurrence after open ventral hernia repairs. J Am Coll Surg 2012;215:787-93. [Crossref] [PubMed]