
Predicting fluid responsiveness with the passive leg raising test: don’t be fooled by intra-abdominal hypertension!
What is passive leg raising (PLR)?
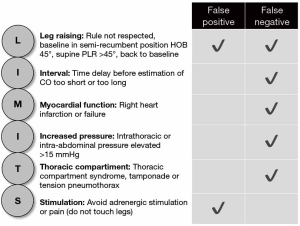
Fluid therapy is often used as first line therapy in critically ill patients in shock. Among the methods currently available to detect preload responsiveness, the PLR test has been demonstrated to be reliable in many studies and a recent meta-analysis (1,2). According to Monnet and Teboul, five rules need to be taken into account, when performing a PLR (3). First, PLR should start from the semi-recumbent and not the supine position. Second, the PLR effects must be assessed by a direct measurement of cardiac output and not by the simple measurement of blood pressure. Third, the technique used to measure cardiac output during PLR must be able to detect short-term and transient changes since the PLR effects may vanish after 1 min. Fourth, cardiac output must be measured not only before and during PLR but also after PLR when the patient has been moved back to the semi-recumbent position, in order to check that it returns to baseline. Fifth, pain, cough, discomfort, and awakening could provoke adrenergic stimulation, resulting in erroneous interpretation of cardiac output changes. We would like to add a sixth rule: confounding factors and underlying conditions that may influence the PLR must be assessed before performing the test, these include increased intrathoracic pressure [high PEEP, presence of autoPEEP, thoracic compartment syndrome (e.g., tension pneumothorax)], cardiac tamponade, right ventricular infarction or failure and intra-abdominal hypertension (IAH), the latter defined as an intra-abdominal pressure (IAP) above 12 mmHg.
The principle of PLR is that a certain volume of blood from the lower extremities and abdominal compartment increases preload and mimics a fluid challenge. During a PLR, on average around 300 mL of blood from the legs and mesenteric splanchnic pool is “autotransfused” to the central circulation. Compared to a fluid bolus or fluid challenge it carries the benefit of not adding additional fluids in case the patient would not be fluid responsive. Indeed, a PLR increases the mean systemic filling pressure (Pmsf) and, in case of preload responsiveness, venous return. In the August issue of Critical Care Medicine, Beurton and colleagues report that IAH can be responsible for a false negative PLR test (4).
We could identify only one other study that addresses the difficulty to assess the hemodynamic status and fluid responsiveness by PLR in patients with IAH. Almost 10 years ago, Mahjoub et al. came to a similar conclusion and found an IAP of ≥16 mmHg responsible for false negative PLR. Hence, they suggested to measure IAP before performing a PLR test (5).
Pooling together all previously described conditions and parameters that influence its sensitivity and specificity, PLR has demonstrated its value as a predictor of fluid responsiveness as long as limitations to its use are understood and respected (Figure 1).
What were the study findings?
The authors collected prospective data on 60 mechanically ventilated patients in whom fluid expansion was planned. After inclusion, they measured a set of hemodynamic parameters, including IAP, in the semi recumbent position. Subsequently, they performed three more measurements: one after the PLR test, the second when the patient was moved back to the semi recumbent position and a last set immediately after a fluid bolus of 500 mL.
The primary goal of the study was to evaluate the PLR test ability to predict fluid responsiveness in patients without IAH (n=30) and in patients with IAH (n=30). The IAP at baseline was 4±3 mmHg in patients without vs. 20±6 mm Hg in patients with IAH (P<0.01).
The area under the receiver operating characteristics (ROC) curve of the PLR test for detecting fluid responsiveness was 0.98±0.02 (P<0.001) in patients without IAH compared to 0.60±0.11 in patients with IAH (P=0.37). In fact, in the IAH group, 21 patients were fluid responders and nine fluid non-responders; among the former group PLR was positive in six patients (true positive) and negative in 15 patients (false negative).
So, why is this study so important?
First, in comparison to the previous study by Mahjoub et al., Beurton et al. have also included fluid non-responsive patients. They assessed CO with the transpulmonary thermodilution technique (PiCCO2 device, Pulsion Medical Systems, Munich, Germany, now fully integrated within Getinge, Sölna, Sweden) instead of an esophageal Doppler probe. Hence, minimizing the inter-operator variability. Moreover, they measured IAP in all different positions during the PLR test.
Second, both studies do not rule out the possibilities to use the PLR in the setting of elevated IAP, but they sure indicate that we need to adjust our common thresholds. In critically ill patients an increase in CI with 15% after fluid bolus is considered as a responder, for a PLR test the threshold is 10%, while it is 5% for the end-expiratory occlusion (7). In 2011, Jacques et al. (8) investigated the dynamic indices of fluid responsiveness [stroke volume variation (SVV), pulse pressure variation (PPV) and systolic pressure variations (SPV)] in nine mechanically-ventilated pigs with induced IAH up to 30 mmHg. They found that respiratory variations in stroke volume and arterial pressure remain indicative of fluid responsiveness but highlighted the fact that threshold values identifying responders and non-responders may be much higher (around 25%) than the usual thresholds of 15% (8). Of course, this data should be translated to critically ill patients with caution but we believe that from a pathophysiological perspective this threshold-adjustment is reasonable (9). To what extent and how proportional adjustments should be made in relation to the actual IAP remains to be clarified by future studies.
Third, these studies encourage us to think about the pathophysiology of increased IAP. We know that PLR results in an endogenous auto-transfusion from the lower part of the body, but we don’t know the exact amount of blood. The blood flows back to the central circulation by gravity during the passive transfer of a patient from the semi recumbent position to a position where the lower limbs are elevated at 45° and the trunk is horizontal. Supposedly, the blood involved comes from the lower limbs and the splanchnic circulation. The decreased effectiveness of PLR during IAH can be explained by a reduction in venous return due to the compression of the portal vein and the inferior vena cava and by a reduction of the abdominal venous capacitance pool due to compression of the splanchnic circulation. Furthermore, one study in animals not only seems to validate this second hypothesis but also highlights the potential role of increased capillary leak in IAH. In a porcine model, 16 pigs were randomly allocated to a control-group or an interventional group; in the latter group IAP was elevated to 15 mmHg by helium insufflation for 120 min followed by further elevation to a level of 30 mmHg for two more hours. The intervention group was associated with increased extravasation of fluid and proteins, plasma volume reduction, reduced cardiac output and reduced perfusion of intra-abdominal organs (10). Therefore, not only we might have a compression of splanchnic circulation but also a direct loss of fluid in the so-called third space, which, to some extent, could affect the volume of blood being auto transfused during PLR. One could argue that, if so, this effect would also be seen in septic shock, which is not the case, in fact PLR is recommended in the Surviving Sepsis Campaign Guidelines as part of the initial hemodynamic management (11). However, those guidelines also advocate the administration of 30 mL/kg fluid starting within the first hour. Overzealous fluid administration may initially sustain intravascular volume. It may also preserve the positive predictive value of PLR (even in capillary leak), but one of the long-term complications this strategy may lead to, is fluid overload. Fluid overload is defined as 10% increase of fluid accumulation from baseline body weight (7). Patients presenting with concomitant heart or kidney failure being much more exposed to this risk.
Fourth, one must not forget physiology. Healthy volunteers with normal heart function are fluid responsive in baseline conditions (on steep part of Frank-Starling curve). Septic shock patients, at the very early stage, may not be hypovolemic but rather vasoplegic, so that instead of fluids, early administration of vasopressors may be more beneficial. Furthermore, fluid responsiveness, too often is associated with the need to administer fluids or the false impression that the patient is hypovolemic. One must always bear in mind that fluid responsiveness may be artificially increased by the heart-lung interactions and the settings of the ventilator. Therefore, studies on the use of PLR must always be interpreted with caution as they may lead to fluid overload.
Finally, and possibly the most interesting result is the significant decrease in IAP during PLR test, both in patients with and without IAH. In analogy to the brain, the abdomen can be considered as a closed box with some parts that are flexible (abdominal wall and diaphragm) and some parts that are rigid (spine, pelvis and costal arch). In analogy to the respiratory system, any change in intra-abdominal volume (IAV) is accompanied by a change in IAP. The relationship between the two is defined as the abdominal (wall) compliance (Caw) (12). Therefore, increased IAP is caused by an increase in IAV (i.e., intra-abdominal bleeding, ascites) or by a decrease in Caw (i.e., retention sutures, tight abdominal closure after laparotomy, obesity, anasarca). During the PLR the diaphragm can be displaced in the cephalic direction thus increasing IAV (more space for same content) and consequently increasing Caw (Figure 2). It seems that this effect played a substantial role in the study by Beurton et al.: in the IAH group, IAP decreased by 29%±11% and in 33% of these patients, IAP decreased below 12 mmHg (the threshold used to define IAH). As addressed by the authors the main limitation is that they could not confirm that these effects persist in the long term. It is however doubtful that this effect might be attenuated if the PLR position is maintained as it seems counterintuitive to put a patient in this position to lower IAP. Other studies have shown that the reverse Trendelenburg position with the abdomen hanging freely may be the best way to unload the thoracic compartment from increased IAP (14-16). Potentially, a measurement error may have occurred with respect to the correct zero reference during the PLR position since one would rather expect an increase in IAP due to the increase in IAV with the blood returning from the legs (more content for same space). Another drawback is that, in mechanical ventilated patients, the increase in IAP would possibly require higher PEEP setting in order to compensate for the cephalic shift of the diaphragm amongst other side effects on other organs from this position (i.e., increased intracranial pressure, atelectasis, possible reflux and ventilated-associated pneumonia).
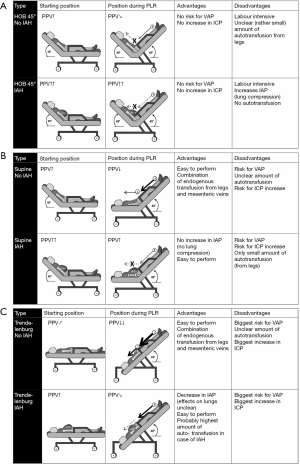
Limitations and cautions
The main limitation of the study is probably its inability to provide an explanation for the false negative PLR test in case of IAH. As underlined by the authors, estimating the transmural pressure gradient of the vena cava by the CVP-IAP gradient is not sufficient enough to investigate this matter; recent methods to enable correct estimation of Pmsf might be more suitable for this purpose (17).
Furthermore, we believe the prognostic value of PLR should be assessed in association with body anthropomorphic data and global indices of perfusion (lactate, base deficit, strong ion difference).
Finally, Beurton et al. study included severely ill patients, mostly septic patients (91%). Only in five patients (8%) the reason of admission was cardiogenic shock. Since IAH worsens all cardiac functions in terms of contractility, preload, afterload and diastolic function (13,14,18), it would have been interesting to analyse separately this subgroup of patients.
Take home message
Be aware that not all blood returns from the legs and the mesenteric splanchnic pool when performing a PLR in patients with increased IAP (19). Therefore, we recommend obtaining a baseline IAP measurement before performing PLR in case of presence of two or more risk factors for IAH. Risk factors for IAH including: decreased Caw, increased IAV (intraluminal or free abdominal) and capillary leak with the use of overzealous fluids. The usual threshold to identify a fluid responder with PLR of 10% may need to be adjusted to 5% (or even less) in case of IAH above 15–20 mmHg. The path ahead is clear and future studies should continue the work previously done. Clinicians dealing with critically ill patients in the ER, OR or ICU should be aware of the pathophysiological effects of increased IAP on the cardiovascular and respiratory system as it has been reported elsewhere (13,20). The incidence of IAH is not uncommon in the ICU as about 25% present with IAH on admission and 50% will develop an IAP above 12 mmHg at some point in time during the first week of stay (21). The Abdominal Compartment Society, formerly known as the World Society of the Abdominal Compartment Syndrome (http://www.wsacs.org) invites interested researchers to join the society and to follow the consensus definitions.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2019.12.14). The series “Hemodynamic Monitoring in Critically Ill Patients” was commissioned by the editorial office without any funding or sponsorship. MLNG Malbrain is a member of the medical advisory Board of Pulsion Medical Systems (now fully integrated in Getinge, Solna, Sweden) and Serenno Medical (Tel Aviv, Israel), consults for Baxter, Maltron, ConvaTec, Acelity, Spiegelberg and Holtech Medical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cherpanath TG, Hirsch A, Geerts BF, et al. Predicting Fluid Responsiveness by Passive Leg Raising: A Systematic Review and Meta-Analysis of 23 Clinical Trials. Crit Care Med 2016;44:981-91. [Crossref] [PubMed]
- Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med 2016;42:1935-47. [Crossref] [PubMed]
- Monnet X, Teboul JL. Passive leg raising: five rules, not a drop of fluid! Crit Care 2015;19:18. [Crossref] [PubMed]
- Beurton A, Teboul JL, Girotto V, et al. Intra-Abdominal Hypertension Is Responsible for False Negatives to the Passive Leg Raising Test. Crit Care Med 2019;47:e639-47. [Crossref] [PubMed]
- Mahjoub Y, Touzeau J, Airapetian N, et al. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med 2010;38:1824-9. [Crossref] [PubMed]
- Michard F, Chemla D, Teboul JL. Applicability of pulse pressure variation: how many shades of grey? Crit Care 2015;19:144. [Crossref] [PubMed]
- Malbrain MLNG, Van Regenmortel N, Saugel B, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D's and the four phases of fluid therapy. Ann Intensive Care 2018;8:66. [Crossref] [PubMed]
- Jacques D, Bendjelid K, Duperret S, et al. Pulse pressure variation and stroke volume variation during increased intra-abdominal pressure: an experimental study. Crit Care 2011;15:R33. [Crossref] [PubMed]
- Tavernier B, Robin E. Assessment of fluid responsiveness during increased intra-abdominal pressure: keep the indices, but change the thresholds. Crit Care 2011;15:134. [Crossref] [PubMed]
- Elvevoll B, Husby P, Øvrebø K, et al. Acute elevation of intra-abdominal pressure contributes to extravascular shift of fluid and proteins in an experimental porcine model. BMC Res Notes 2014;7:738. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Malbrain ML, Peeters Y, Wise R. The neglected role of abdominal compliance in organ-organ interactions. Crit Care 2016;20:67. [Crossref] [PubMed]
- Malbrain ML, De Waele JJ, De Keulenaer BL. What every ICU clinician needs to know about the cardiovascular effects caused by abdominal hypertension. Anaesthesiology Intensive Therapy 2015;47:388-99. [Crossref] [PubMed]
- Reuter DA, Felbinger TW, Schmidt C, et al. Trendelenburg positioning after cardiac surgery: effects on intrathoracic blood volume index and cardiac performance. Eur J Anaesthesiol 2003;20:17-20. [Crossref] [PubMed]
- Malbrain MLNG, Reuter DA. Assessing fluid responsiveness with the passive leg raising maneuver in patients with increased intra-abdominal pressure: Be aware that not all blood returns. Critical Care Medicine 2010;38:1912-5. [Crossref] [PubMed]
- Wilcox S, Vandam LD. Alas, poor Trendelenburg and his position! A critique of its uses and effectiveness. Anesth Analg 1988;67:574-8. [Crossref] [PubMed]
- Wijnberge M, Sindhunata DP, Pinsky MR, et al. Estimating mean circulatory filling pressure in clinical practice: a systematic review comparing three bedside methods in the critically ill. Ann Intensive Care 2018;8:73. [Crossref] [PubMed]
- Cheatham ML, Malbrain ML. Cardiovascular implications of abdominal compartment syndrome. Acta Clin Belg 2007;62 Suppl 1:98-112. [Crossref]
- Robotham JL, Wise RA, Bromberger-Barnea B. Effects of changes in abdominal pressure on left ventricular performance and regional blood flow. Crit Care Med 1985;13:803-9. [Crossref] [PubMed]
- Regli A, Pelosi P, Malbrain M. Ventilation in patients with intra-abdominal hypertension: what every critical care physician needs to know. Ann Intensive Care 2019;9:52. [Crossref] [PubMed]
- Malbrain ML, Chiumello D, Cesana BM, et al. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol 2014;80:293-306. [PubMed]


