
Expression of HMGB1 and TLR4 in neuropsychiatric systemic lupus erythematosus patients with seizure disorders
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease characterized by the formation of autoantibodies and inflammation in multiple organs (1). Neuropsychiatric SLE (NPSLE) is a form of SLE associated with severe neuropsychiatric (NP) syndromes, including various neurological and psychiatric features (2). Several studies estimated that between 12% and 95% of their sample SLE patients had NPSLE (1,3-5). The long-term prognosis of SLE patients indicates that seizures are among the most ominous and clinical manifestations of damage accrual by NPSLE (6). However, the pathogenesis of NPSLE related seizures has not yet been elucidated.
The high mobility group box 1 (HMGB1) is a highly conserved nuclear DNA-binding protein released by innate immune cells in pathological conditions (7). Recent studies revealed increased expression levels of serum HMGB1 in SLE patients, indicating that HMGB1 could be a marker of active SLE (8). The toll-like receptor 4 (TLR4) is an endogenous pattern recognition receptor of HMGB1, and it mediates chronic inflammatory and autoimmune diseases (9-12). Also, TLR4 has been shown to facilitate SLE pathogenesis by regulating T cells and inducing autoantibodies (13). Meanwhile, HMGB1 and TLR4 contribute to the occurrence and persistence of seizures. For instance, HMGB1 is an epilepsy-associated cytokine since the pathophysiology of epilepsy indicates that HMGB1 increase in quantity and distribution intensifies seizures. Previous studies observed that susceptibility to seizures was reduced in TLR4 knockout mice (14,15). Using mice models, the study by Mattia et al. revealed that antagonists of HMGB1 and TLR4 retard seizure occurrence and reduced recurrence of acute and chronic seizures (16). However, previous researches on the effects of HMGB1 and TLR4 on seizure disorders involved primary or drug-induced epilepsy, not SLE.
Since HMGB1 and TLR4 participate in both SLE and seizures, it is crucial to determine whether HMGB1 and TLR4 play any role in NPSLE related seizures. Currently, few clinical studies have explored this subject. Besides, two studies examined TLR4 gene polymorphism in NPSLE patients but gave inconsistent conclusions. A study by Bogaczewicz et al. reported no correlation between TLR4 polymorphism and NPSLE in a Polish population (17). On the contrary, a study done in South India reported a positive association between the TLR4 polymorphism T399I and NPSLE related seizures (18). Therefore, this study explored the expression of HMGB1 and TLR4 in a large group of SLE patients. We evaluated the correlation between disease activity and HMGB1/TLR4 expression, focusing on involvement in neuropsychiatric syndromes, particularly NPSLE related seizures.
Methods
Patients and samples
We prospectively enrolled 291 SLE patients who visited the Department of Rheumatology, Nanfang Hospital, Guangzhou, China, from January 2013 to June 2018. Patients who were older than 14 years and met four of the 1997 revised classification criteria of the American College of Rheumatology (ACR) were eligible for inclusion (19). Patients who had other autoimmune diseases were excluded. The SLE disease activity index 2000 (SLEDAI-2k) was used to assess disease activity patterns in patients (20) with an SLE disease activity of SLEDAI-2k <4 was considered quiescent, while the activity of SLEDAI-2k ≥4 was deemed to be active. The active disease cohort included 188 patients, while the quiescent cohort included 103 patients. Active SLE patients were classified into two groups, NPSLE (N=86) and Non-NPSLE (N=102) groups. The NPSLE definition was based on the 1999 ACR nomenclature and case definitions for neuropsychiatric lupus syndromes (21). Patients who developed neuropsychiatric syndromes not attributable to SLE (electrolyte imbalances, infections or medications) were excluded. Meanwhile, 100 age- and gender-matched healthy controls (HC) were recruited from the Physical Examination Center of Nanfang Hospital.
Clinical data were collected from all patients, and the SLE serologic variables of complement component 3 (C3) and complement component 4 (C4) were recorded. Also recorded were the antibody levels of anti-double-stranded DNA (anti-dsDNA), anti-ribosomal P protein (anti-rib-P), anti-SSA, anti-SSB, anti-cardiolipin (Acl), and anti-β2 glycoprotein I (β2-GPI). All the antibodies were detected at the clinical laboratory of Nanfang Hospital. The neuropsychiatric (NP) syndromes and cerebrospinal fluid (CSF) examination results of NPSLE patients were also recorded.
A venous puncture was done, and 2 mL of blood was collected in a serum separator tube. The blood was maintained at room temperature for 20 min to allow for complete coagulation and serum was separated by centrifugation at 1,000 ×g for 10 min. The serum was stored at −80 °C in polypropylene tubes until further use. Blood (3 mL) for RNA extraction was collected in a vacutainer tube containing 15% EDTA solution. Peripheral blood monocytes (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation and stored at −80 °C until use for RNA extraction. Cerebrospinal fluid (CSF) samples were collected from the NPSLE patients and stored at −80 °C until the biomarker assays were performed.
Measurement of serum and CSF HMGB1
Serum and CSF levels of HMGB1 were determined using an HMGB1 ELISA kit (Shino-Test Corp., Tokyo, Japan) according to the manufacturer’s guidelines. In short, a 96-well plate was coated with samples (10 µL/well) in duplicate and incubated at 37 °C for 20 h. The plate was then washed five times in wash buffer and dried by gentle on a lint-free paper towel. Subsequently, 100 µL of the detection antibody solution was added to each well, and the plate was incubated for 2 h at room temperature. An equal volume (100 µL/well) of the substrate solution was then added and incubated at room temperature for 30 min, protected from light. The reaction was stopped by the addition of 100 µL/well of 1 M H2SO4, and the optical density (OD) was determined at a wavelength of 450 nm 5 min later. Results were fitted to the standard curve, and the HMGB1detection range was 0.2–80 ng/mL.
Expression of TLR4 in PBMCs
TLR4 mRNA was extracted from PBMCs using TRizol Reagent (Invitrogen, Carlsbad, CA). The total PBMC samples of 291 patients and 100 samples of HC in each group were divided into several subgroups before RNA extraction. In each subset, a certain amount (1×106 cells) of the PBMC samples were mixed into one. In total, we had 19 PBMC samples of active SLE (NPLSE: n=9; non-NPSLE: n=10), 10 of quiescent SLE, and 10 of the HCs. Similarly, we divided the PBMC samples of 21 NPSLE with seizure and 65 without seizure into seven subgroups, separately. The concentration of total RNA was quantified using a nanodrop spectrophotometer (Nanodrop 1000; Thermo Scientific). Complementary DNA (cDNA) was synthesized from 200 ng of RNA using random primers and Super-Script II reverse transcriptase (Invitrogen) according to the manufacturer’s instruction. Synthesized cDNAs were used as templates for quantitative real-time PCR analysis using TaqMan gene expression assays (Taq-Man; Applied Biosystems). Relative gene expression levels were determined after normalizing cycle thresholds against the β-actin housekeeping gene and presented as the relative fold change by the comparative Ct (2–ΔΔCt) method.
Statistical analysis
Data were expressed as means ± standard deviation (SD). Clinical and laboratory variables were compared between groups using the t-test for normally distributed variables, and the Mann-Whitney U test for non-normally distributed data. Differences among observed frequencies were tested using the Chi-square test, while Pearson’s correlation coefficient was used to calculate the correlation between variables. The independent NPSLE variables were predicted by binary logistic regression. The sensitivity and specificity of HMGB1 serum of various patients were determined from the area under the curve (AUC) of the receiver-operating characteristic (ROC) curve and Youden index (YI). A P value <0.05 was considered statistically significant. The GraphPad Prism 7.0a (GraphPad Software Inc., San Diego, CA, USA) and SPSS Statistics 24.0 (IBM Corp, Armonk, NY, USA) software were used for statistical analysis.
Results
Clinical characteristics and laboratory findings of study objects
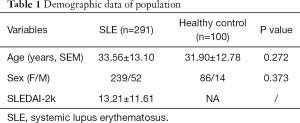
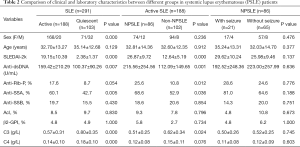
The clinical characteristics and laboratory findings of the study objects are summarized in Tables 1,2. The mean age of SLE patients (239 females and 52 males) was 33.56±13.10 years. The healthy control (HC) group comprised 100 volunteers (86 females and 14 males), and the mean age was 31.90±12.78 years (Table 1). A total of 188 SLE patients had active disease, while the 103 SLE patients were quiescent. Significant differences were obtained in gender (P=0.000), anti-SSA antibody (P=0.005), anti-dsDNA antibody (P=0.007), SLEDAI-2k (P=0.000), C3 (P=0.000), and C4 (P=0.000) between the active and quiescent groups of SLE patients. Out of 188 active SLE patients, 86 had neuropsychiatric manifestations. The mean age of NPSLE patients (74 females and 12 males) was 32.81±14.36 years. Levels of anti-dsDNA antibody (P=0.001) and SLEDAI-2k (P=0.000) were significantly higher, while C3 levels were significantly lower (P=0.024) in NPSLE than in non-NPSLE group. Furthermore, anti-rib-P antibody (+) (P=0.012) and anti-SSA antibody (+) (P=0.036) were detected more frequently in NPSLE group samples compared with non-NPSLE group samples. Out of 86 NPSLE patients, 21 had seizure disorders. The occurrence frequency of autoantibodies between the NPSLE with seizure group and without seizure group was comparable. The levels of C3, C4, and SLEDAI-2k between the two groups were equally comparable (Table 2).
Full table
Full table
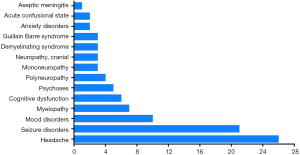
The occurrence of NP disease was classified according to the ACR case definitions for NPSLE, including detailed diagnostic guidelines for 19 NP syndromes (21). A total of 14 NP syndromes were identified, and 96 NPSLE events were observed in the 86 NPSLE patients (Figure 1). Among these NP events, central nervous system (CNS) manifestations accounted for 96.5% (83/86 patients), while the involvement of the peripheral nervous system (PNS) was 15.1% (13/86 patients). The majority of NP symptoms were headache (n=26; 30.2%), seizure disorders (n=21; 24.4%), mood disorders (n=10; 11.6%),myelopathy (n=7; 8.1%) and cognitive dysfunction (n=6; 7.0%).
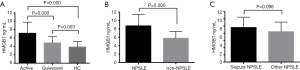
Serum HMGB1 levels in the investigated groups
Serum levels of HMGB1 in quiescent SLE patients were significantly higher (4.84±0.15 ng/mL) compared to the control group (3.86±0.13 ng/mL), while those in active SLE patients were the highest (7.14±0.19 ng/mL) (Figure 2A). Active SLE patients with NP manifestations showed higher HMGB1 levels (8.73±0.29 ng/mL) compared to active patients without NP manifestations (5.79±0.16 ng/mL) (Figure 2B). Comparable expression levels of serum HMGB1 were found in NPSLE patients with seizure and without seizure disorders (9.59±0.63 and 8.45±0.33 ng/mL, respectively) (Figure 2C).
CSF HMGB1 levels in the NPSLE group
The present study obtained 59 CSF samples from NPSLE patients, 21 samples with seizure, and 38 without seizure disorders. The CSF white blood cell count of the two groups was comparable (1.10±1.51 and 2.02±2.23, respectively) (Figure 3A). However, the CSF protein was significantly higher in the seizure NPSLE group (1.00±0.81 g/L) than in the NPSLE group without seizure disorders (0.41±0.56 g/L) (Figure 3B). A similar expression of CSF HMGB1 was observed in the two groups (2.90±2.29 and 2.56±1.70 ng/mL, respectively) (Figure 3C).

Correlation between serum HMGB1 levels and SLE disease activity
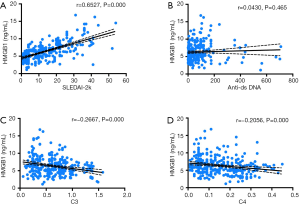
Since HMGB1 is reported to be a potential biomarker for SLE disease activity, this study evaluated the correlation between serum HMGB1 levels and various serological parameters. We observed a significant correlation between HMGB1 levels and SLEDAI-2k (r=0.6527, P=0.000) (Figure 4A). We also investigated the correlation between anti-ds DNA antibody and HMGB1 levels. However, no significant correlation was noted between the two parameters (r=0.0430, P=0.465) (Figure 4B). The findings of our study also suggest that serum HMGB1 levels are negatively correlated with C3 (r=−0.2667, P=0.000) and C4 (r=−0.2056, P=0.000) (Figure 4C,D).
The mRNA expression of TLR4 in PBMC in the investigated groups
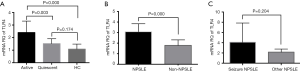
The mRNA levels of TLR4 in SLE patients with active disease were higher than those of patients with the quiescent illness, as well as HC (P=0.003 and P=0.000, respectively) (Figure 5A). The mRNA levels of TLR4 were also higher in the NPSLE group compared to the Non-NPSLE group (P=0.000) (Figure 5B). Notably, mRNA expression of TLR4 was comparable between the NPSLE with the seizure group and the group without seizure disorders (Figure 5C).
Prediction of NPSLE
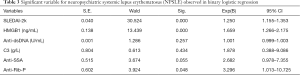
Our study results indicate that SLEDAI-2k, HMGB1, anti-ds DNA Ab, C3, anti-SSA Ab, and anti-rib-P Ab are significantly associated with NPSLE. These factors were subjected to binary logistic regression to predict NPSLE. According to the results of the binary logistic regression, variables independently associated with the occurrence of NPSLE include SLEDAI-2k, serum HMGB1and anti-Rib-P Ab. The odds ratios (ORs) of the above independent variables are 1.250 (95% CI: 1.155–1.353; P=0.000), 1.659 (95% CI: 1.266–2.175; P=0.000) and 3.296 (95% CI: 1.013–10.725; P=0.048), respectively (Table 3).
Full table
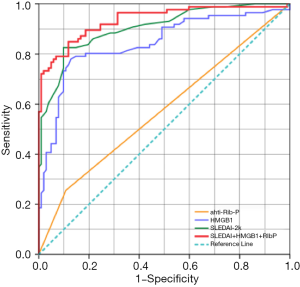
The ROC curves for serum HMGB1 and other variables in NPSLE are shown in Figure 6. ROC analysis of serum HMGB1, SLEDAI, and anti-rib-p Ab, and their correlation to NPSLE reveals substantial prediction of NPSLE. The above independent variables had an AUC (95% CI) of 0.843 (0.783–0.903), 0.905 (0.862–0.948), and 0.574 (0.491–0.657), respectively (P<0.05). Notably, ROC analysis for the total risk of the combination of serum HMGB1, SLEDAI, and anti-rib-p Ab had a larger AUC (95% CI) of 0.936 (0.900–0.971) (P<0.0001), indicating an excellent prediction of NPSLE occurrence.
Discussion
The present study reveals three main findings: (I) Serum HMGB1 levels are increased in SLE patients, especially in NPSLE, and therefore correlated with disease activity; (II) the gene expression of TLR4 mRNA in PBMCs is increased in NPSLE and (III) HMGB1 and TLR4 have minimal effect on NPSLE related seizures.
To our knowledge, the present study is the first report to examine the levels of HMGB1 in NPSLE patients. HMGB1 is a crucial gene expressed in the nucleus, and its translocation to the extracellular matrix is a vital warning signal of autoimmune diseases (7). Apart from autoimmune diseases, the expression of HMGB1 is increased in nervous system diseases such as cerebral ischemia, atherosclerosis, and ischemia-reperfusion injury (22-24). Consistent with previous reports (25,26), our study found that HMGB1 concentrations in serum were higher in SLE patients than in healthy controls. Moreover, levels of circulating HMGB1 in SLE patients were positively correlated to SLEDAI-2k and negatively correlated with C3 and C4, suggesting that HMGB1 may be involved in the inflammatory process of SLE. However, no previous study has explored the correlation between HMGB1 and NPSLE. The present study observed that levels of serum HMGB1 are increased in NPSLE patients and are positively associated with disease activity. Our logistic regression and ROC analysis results indicate that serum HMGB1 is independently related to the occurrence of NPSLE. We postulated that the role of HMGB1 in NPSLE might be based on its common effects on autoimmune and nervous system diseases, such as triggering the release of pro-inflammatory factors and promoting neuronal differentiation of brain cells. However, the specific mechanism needs to be explored further.
The second main finding of the present study is that the mRNA expression of TLR4 in PBMCs was increased in NPSLE. In recent years, studies have shown that TLR4 signaling is a potent trigger for SLE. It was observed that Chaperonin 10, a TLR4 signaling inhibitor can efficiently inhibit cutaneous lupus and lupus nephritis (27,28). Furthermore, compared with TLR4-producing SLE model mice, the titers of anti-nuclear, anti-ds DNA and Acl antibodies were decreased in TLR4-deficient SLE model mice (29). However, few studies have reported on the correlation between TLR4 and NPSLE. Also, only two of these reports examined TLR4 gene polymorphism, and the findings of the two studies were inconsistent because of differences in population dynamics. No correlation was found between CC and CT genotypes of TLR4 1196C/T and NPSLE in a Polish population. In a South Indian population, however, the TLR4 polymorphism T399I was found to be positively correlated with NPSLE related seizures (17,18).
The last main finding of our study is that HMGB1 and TLR4 have minimal effect on NPSLE related seizures. Seizures are one of the most severe complications of NPSLE and can occur at any time in the course of SLE (30). In the last decade, research interest in the HMGB1-TLR4 pathway in SLE and seizure disorders has seen significant growth separately. An in vitro study demonstrated that the HMGB1-TLR4 pathway upregulates apoptosis in macrophages by triggering nuclear translocation of NF-kB in a MyD88-dependent mechanism. And this immune regulatory pathway plays a vital role in the pathogenesis of SLE (31). The CSF concentrations of HMGB1, IL-6, and IL-17A were increased in patients with autoimmune encephalitis. This increase suggests that CSF HMGB1 could be an essential factor promoting inflammatory responses in autoimmune diseases that involve the CNS (32).
Meanwhile, HMGB1-induced neuronal hyperexcitability on seizure models is mediated by activation of neuronal TLR4 and N-methyl-D-aspartate (NMDA) receptors, which promote calcium influx in pyramidal neuron cell bodies (33,34). Our previous study conducted on 36 NPSLE patients and 37 Non-NPSLE patients showed increased expression of anti-NMDA receptor antibodies in the NPSLE group (35). In this study, the levels of both serum and CSF HMGB1 in NPSLE with seizure were higher than in patients with other neuropsychiatric syndromes, but without significance. The serum TLR4 mRNA expression levels in PBMCs were similar in the NPSLE with seizure and without seizure group. Although HMGB1-TLR4 participated in both NPSLE and seizures, they had little effect on NPSLE related seizures, which implies different pathophysiology between epilepsy and NPSLE related seizures.
The clinical manifestations of NPSLE are diverse and highly heterogeneous in severity and prognosis, and the precise pathophysiology of NPSLE remains poorly understood. Several immune factors that facilitate NPSLE pathogenesis, such as cytokines and brain-reactive autoantibodies among others, were evaluated. One possible explanation is that the neuronal damage in NPSLE may be caused by the cross-reaction between anti-nuclear antibody and neuronal antigens, resulting from the direct action of anti-neuronal antibodies or immune complex-mediated vasculitis (36). So far, 20 autoantibodies (eleven brain-specific antibodies and nine systemic antibodies) have been identified to be associated with NPSLE (37). In this study, we found that anti-SSA and anti-rib-P antibodies are associated with NPSLE. And anti-rib-P antibody is the independent predictor of NPSLE occurrence. Our findings are consistent with other studies (38,39). However, the antibody occurrence frequency was not significantly different between the NPSLE with seizures and without seizures group. Therefore, which autoantibody contributes to which type of NPSLE is still elusive so far.
Our study is not without limitations. First, the number of patients with NPSLE is small, especially those with seizures. Therefore, these preliminary findings should be validated in a larger sample size of NPSLE patients, covering all phenotypes. Secondly, the expression of HMGB1 and TLR4 was measured at the time of entry, and therefore changes in the expression of these proteins before and after treatment remain unknown in the current study. Thirdly, did not examine the relative quantities of inflammatory mediators (interleukins, TNF-α, IFN-γ, etc.), which are considered to be crucial in HMGB1-TLR4 signaling pathway; thus, potential mechanisms involving pathways such as the HMGB1-TLR4 signaling pathway and NPSLE need to be explored in future studies.
Conclusions
This study demonstrated that the expression of HMGB1 and TLR4 was increased in NPSLE, although HMGB1 and TLR4 had minimal effect on NPSLE related seizures. The serum levels of HMGB1 were positively correlated with disease activity, which could be a potential biomarker of NPSLE. Serum HMGB1 can, therefore, be used for clinical practice in the future.
Acknowledgments
Funding: This work was supported by Grant from the National Natural Science Foundation of China (No.81801624), and the grant from Medical Scientific Research Foundation of Guangdong Province of China (No. A2019020).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was in compliance with the Declaration of Helsinki and approved by the ethics committee of Nanfang Hospital, Southern Medical University.
References
- Bertsias GK, Boumpas DT. Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat Rev Rheumatol 2010;6:358-67. [Crossref] [PubMed]
- Jarpa E, Babul M, Calderon J, et al. Common mental disorders and psychological distress in systemic lupus erythematosus are not associated with disease activity. Lupus 2011;20:58-66. [Crossref] [PubMed]
- Unterman A, Nolte JE, Boaz M, et al. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin Arthritis Rheum 2011;41:1-11. [Crossref] [PubMed]
- Ainiala H, Loukkola J, Peltola J, et al. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology 2001;57:496-500. [Crossref] [PubMed]
- Borowoy AM, Pope JE, Silverman E, et al. Neuropsychiatric lupus: the prevalence and autoantibody associations depend on the definition: results from the 1000 faces of lupus cohort. Semin Arthritis Rheum 2012;42:179-85. [Crossref] [PubMed]
- Andrade RM, Alarcón GS, González LA, et al. Seizures in patients with systemic lupus erythematosus: data from LUMINA, a multiethnic cohort (LUMINA LIV). Ann Rheum Dis 2008;67:829-34. [Crossref] [PubMed]
- Harris HE, Andersson U, Pisetsky DS. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol 2012;8:195-202. [Crossref] [PubMed]
- Li J, Xie H, Wen T, et al. Expression of high mobility group box chromosomal protein 1 and its modulating effects on downstream cytokines in systemic lupus erythematosus. J Rheumatol 2010;37:766-75. [Crossref] [PubMed]
- Tsung A, Klune JR, Zhang X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 2007;204:2913-23. [Crossref] [PubMed]
- Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 2007;220:47-59. [Crossref] [PubMed]
- Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007;13:1050-59. [Crossref] [PubMed]
- Fan J, Li Y, Levy RM, et al. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: Role of HMGB1-TLR4 signaling. J Immunol 2007;178:6573-80. [Crossref] [PubMed]
- Liu B, Yang Y, Dai J, et al. TLR4 up-regulation at protein or gene level is pathogenic for lupus-like autoimmune disease. J Immunol 2006;177:6880-8. [Crossref] [PubMed]
- Huang JS, Wu Y, Huang Q, et al. Expression level and distribution of HMGB1 in Sombati's cell model and kainic acid-induced epilepsy model. Eur Rev Med Pharmacol Sci 2015;19:2928-33. [PubMed]
- Iori V, Maroso M, Rizzi M, et al. Receptor for advanced glycation endproducts is upregulated in temporal lobe epilepsy and contributes to experimental seizures. Neurobiol Dis 2013;58:102-14. [Crossref] [PubMed]
- Maroso M, Balosso S, Ravizza T, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 2010;16:413-9. [Crossref] [PubMed]
- Bogaczewicz A, Sobow T, Bogaczewicz J, et al. Toll-like receptor 4 gene polymorphism 1196 C/T does not influence the risk of neuropsychiatric systemic lupus erythematosus in Polish population--a preliminary report. Lupus 2013;22:1504-8. [Crossref] [PubMed]
- Rupasree Y, Naushad SM, Rajasekhar L, et al. Association of TLR4 (D299G, T399I), TLR9 1486T>C, TIRAP S180L and TNF-a promoter (1031, 863, 857) polymorphisms with risk for systemic lupus erythematosus among South Indians. Lupus 2015;24:50-7. [Crossref] [PubMed]
- Smith EL, Shmerling RH. The American College of Rheumatology criteria for the classification of systemic lupus erythematosus: Strengths, weaknesses, and opportunities for improvement. Lupus 1999;8:586-95. [Crossref] [PubMed]
- Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288-91. [PubMed]
- Hanly JG. ACR classification criteria for systemic lupus erythematosus: Limitations and revisions to neuropsychiatric variables. Lupus 2004;13:861-4. [Crossref] [PubMed]
- Goldstein RS, Gallowitsch-Puerta M, Yang L, et al. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock 2006;25:571-4. [Crossref] [PubMed]
- Porto A, Palumbo R, Pieroni M, et al. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J 2006;20:2565-6. [Crossref] [PubMed]
- Vadacca M, Buzzulini F, Rigon A, et al. Neuropsychiatric lupus erythematosus. Reumatismo 2006;58:177-86. [PubMed]
- Urbonaviciute V, Fürnrohr BG, Weber C, et al. Factors masking HMGB1 in human serum and plasma. J Leukoc Biol 2007;81:67-74. [Crossref] [PubMed]
- Jiang W, Pisetsky DS. Expression of high mobility group protein 1 in the sera of patients and mice with systemic lupus erythematosus. Ann Rheum Dis 2008;67:727-8. [Crossref] [PubMed]
- Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 2006;6:823-35. [Crossref] [PubMed]
- Kulkarni OP, Ryu M, Kantner C, et al. Recombinant chaperonin 10 suppresses cutaneous lupus and lupus nephritis in MRL-(Fas)lpr mice. Nephrol Dial Transplant 2012;27:1358-67. [Crossref] [PubMed]
- Lartigue A, Colliou N, Calbo S, et al. Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. J Immunol 2009;183:6207-16. [Crossref] [PubMed]
- Mackworth-Young CG, Hughes GR. Epilepsy: an early symptom of systemic lupus erythematosus. J Neurol Neurosurg Psychiatry 1985;48:185. [Crossref] [PubMed]
- Yang H, Hreggvidsdottir HS, Palmblad K, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A 2010;107:11942-7. [Crossref] [PubMed]
- Ai P, Zhang X, Xie Z, et al. The HMGB1 is increased in CSF of patients with an Anti-NMDAR encephalitis. Acta Neurol Scand 2018;137:277-82. [Crossref] [PubMed]
- Walker LE, Frigerio F, Ravizza T, et al. Molecular isoforms of high-mobility group box 1 are mechanistic biomarkers for epilepsy. J Clin Invest 2019;129:2166. [Crossref] [PubMed]
- Balosso S, Liu J, Bianchi ME, et al. Disulfide-containing high mobility group box-1 promotes N-Methyl-D-Aspartate receptor function and excitotoxicity by activating toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid Redox Signal 2014;21:1726-40. [Crossref] [PubMed]
- Yang Y, Yuan C, Shen S, et al. Autoantibodies to NR2A peptide of the Glutamate/NMDA receptor in patients with seizure disorders in neuropsychiatric systemic lupus erythematosus. Mediators Inflamm 2017;2017:5047898. [Crossref] [PubMed]
- Kawakami T, Yamazaki M, Takakuwa Y, et al. Microscopic polyangiitis associated with antiphospholipid antibodies and immune complex mediated cutaneous vasculitis. Acta Derm Venereol 2010;90:639-41. [Crossref] [PubMed]
- Zandman-Goddard G, Chapman J, Shoenfeld Y. Autoantibodies involved in neuropsychiatric SLE and antiphospholipid syndrome. Semin Arthritis Rheum 2007;36:297-315. [Crossref] [PubMed]
- Sciascia S, Bertolaccini ML, Roccatello D, et al. Autoantibodies involved in neuropsychiatric manifestations associated with systemic lupus erythematosus: a systematic review. J Neurol 2014;261:1706-14. [Crossref] [PubMed]
- Arinuma Y, Kikuchi H, Hirohata S. Anti-ribosomal P protein antibodies influence mortality of patients with diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematous involving a severe form of the disease. Mod Rheumatol 2019;29:612-8. [Crossref] [PubMed]


