Structural analysis of glucans
Introduction
Polymers of glucose, glucans, with different types of glycosidic linkages and anomeric configurations are most widespread polysaccharides in the nature. Most of them play a role of cell wall structural components; others are used as the energetic source for metabolism. Despite simple monosaccharidic composition, glucans demonstrate large structural variability. They vary in anomeric configuration of d-Glcp units, position and distribution of glycosidic bonds, molecular size and type and degree of branching (DB). There are three main structural types of these polysaccharides, namely α-glucans, β-glucans and mixed α,β-glucans (1). The configuration of glycosidic bonds and molecular mass are also important for glucan characterization. Structures, sources and physiological function of common and some unusual glucans are summarized in Table 1.
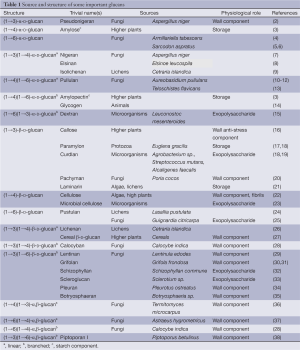
Full table
Linear (1→4)-α-D-glucans and branched (1→4) (1→6)-α-D-glucans are widespread in the nature playing a role of energy supply. These glucans represent basic structures of amylose and amylopectin, the polysaccharidic components of starch in plants (3). Animal glycogen (12) as well as some fungal polysaccharides are also branched (1→4) (1→6)-α-D-glucans. Unlike it, pullulan, a water-soluble fungal polysaccharide, is linear glucan containing both α-(1→4) and α-(1→6) linkages (10). It consists of regularly repeating maltotriose and, in some cases, maltotetraose fragments connected by α-(1→6) glycosidic bonds (11).
Cellulose, the linear (1→4)-β-D-glucan, is the most abundant polysaccharide (22). It constitutes mainly plant cell walls and derived materials like wood or fibers (cotton, flax, etc.). The cellulose chains are aggregated into microfibrils or more complex ordered structures stabilized by intra- and intermolecular hydrogen bonds (39). These microfibrils are primary architectonical elements of cell walls of higher plants and algae. Rigid cellulose fibrils are embedded in amorphous polymers like neutral and acidic polysaccharides, proteins, and aromatics. Cellulose is also produced by certain microorganisms and can be obtained by fermentation (40). In addition to cellulose, linear mixed (1→4) (1→3)-linked β-D-glucans, in which blocks of (1→4)-linked β-Glcp units are separated by single (1→3)-linkages, are widely present in plants, mainly cereals (41). Most of these cellulose-like segments are tri- and tetramers, but longer (1→4)-linked oligomers may also be present in the chains. Cereal β-glucans from different sources vary in their tri- to tetramer ratio, the share of longer cellulosic fragments and the ratio between two types of glycosidic bonds (42).
Fungal cell walls, which consist mainly of structural polysaccharides and glycoproteins, are the main source of various structural types of glucans (1). There are many fungal and microbial polysaccharides containing β-(1→3)- and/or β-(1→6)-linked β-D-Glcp units, and β-(1→4)-linked β-D-Glcp residues may be also present (43,44). These β-glucans commonly have trivial names according their fungal origin (grifolan, lentinan, pachyman, pleuran, schizophylan, scleroglucan, etc.). Besides that differently composed fungal glucans have been described, including linear alkali soluble (1→3)-α-D-glucans (45-47) and linear or branched α,β-glucans (28,36-38). Fungal glucans often forms various complexes with other polysaccharides, proteins or phenolics.
Isolation of glucans from raw materials includes various extraction and purification procedures. The check of purity helps in evaluation of the crude polysaccharidic fractions and isolation/purification steps. More rigorous analyses are required to clarify the structure of purified glucans. Modern chemical, spectroscopic and separation methods are used to solve these tasks. In the following paragraphs analytical methods commonly used in the structural analysis of glucans are reviewed.
Purity
Before rigorous structural analyses of isolated glucan it is necessary to estimate its purity, i.e., the proportion of polysaccharide component and the presence of impurities. Total and/or reducing sugar assays as well as testing of other compounds occurrence are usually used to solve this task. The term “total sugars” covers all mono-, oligo- and polysaccharides in comparison with non-sugars (proteins, lipids, aromatics, mineral compounds, etc.) that may be present in the glucan preparations. In a pure polysaccharide, concentration of reducing sugars, having free carbonyl group at the reducing ends, represents quantity of reducing-end groups, depending on polymerization degrees (DP) and DB. Estimation of linkages and structures, which are typical for ballast polysaccharides including non-targeted glucans, is the next analytical task. Methods commonly used for these purposes are summarized in Table 2.
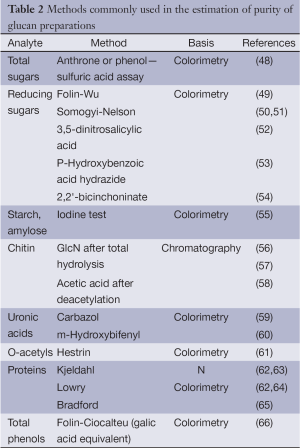
Full table
Molecular mass
Weight average molecular mass (Mw), z-average radius of gyration (Rg), intrinsic viscosity ([η]) and other molecular characteristics of soluble glucans can be determined by size-exclusion (gel permeation) chromatography (SEC or GPC), laser light scattering (LLS) and viscometry. Polysaccharides are commonly dissolved in aqueous system or analyzed directly in the extracts. Problems in analysis of insoluble or partially soluble glucans can be overcome by specific solvent systems or appropriate chemical modification. Structural homogeneity, branching, molecular weight distribution and aggregation-disaggregation of glucans can be estimated by combination of these methods.
Molecular characteristics (Mw, Rg) of the amylose and amylopectin fractions of corn starch were estimated by high-performance SEC (HPSEC) with multi-angle laser-light scattering (MALLS) and refractive index (RI) detectors (67). The specific volume for gyration (SVg) was 0.529 for amylose and 0.092 for amylopectin, respectively. This value was used for comparison of the molecular compactness or DB. DMSO/water and DMSO/LiBr systems were used as solvents for MALLS batch mode Mw analysis of rice starch, amylose and amylopectin (68). The former system demonstrated better ability to dissolve starch and reduce starch aggregation. Shear degradation of native starch was examined by SEC using DMSO/LiBr systems as eluent (69). Extensive shear scission was observed only in the amylopectin region in the size distribution/flow rate curve. Authors suggested that this SEC system permits to obtain the complete size distribution only for smaller sizes, primarily amylose. Starches with extreme phosphate content were analyzed by SEC using NaOH solutions for starch dissolving and elution (70). Starch aggregation was estimated by on line RI and MALLS detection. Three major regions in the SEC profile were identified as amylopectin aggregates, amylopectin and amylose. Starches with a high amount of amylopectin aggregates showed high peak viscosities. Extreme amount of either starch bound phosphate or amylose suppresses aggregation and thus decreases viscosity. A N,N-dimethylacetamide/LiCl system was used instead of water as solvent and mobile phase for SEC for pullulan and other glucans (71). Pure and aqueous cadoxen was used in LLS of linear (1→3)-β-D-glucan from Poria cocos (72). This polysaccharide was found to form aggregates (206 kDa) in the cadoxen-water mixtures, while single-stranded chains (89.3 kDa) were detected in pure cadoxen. SEC study of this glucan in DMSO/LiCl system confirmed partial temperature sensitive aggregation that was inhibited by heating to 80 °C (73). SEC combined with LLS (SEC-LLS) and viscometry in the same solution was used in the study of linear (1→3)-α-D-glucan from Poria cocos (46). At the measuring conditions this polysaccharide showed relatively extended flexible chain conformation. Disaggregation of similar (1→3)-α-D-glucan from Lentinus edodes was analyzed by LLS in an aqueous NaOH/urea system (47). It was found that stronger conditions led to a partial hydrolysis of this polysaccharide.
Chemolytic methods
Chemolytic analysis of glucans is based on chemical or enzymatic cleavage of this polysaccharide followed by separation of degradation products (oligo- or monosaccharides or even smaller fragments). A parent glucan and/or degradation products can be chemically modified for analytical purposes. Resistance of glycosidic bonds is not the same for various glucans and depends on anomeric configuration, position, molecular environment and availability. Chemolytic methods are still attractive for linkage analysis of complex glucans.
Total hydrolysis of glucans can be made under strong acidic conditions by the use of formic, sulfuric or trifluoroacetic acids at concentrations around 2 mol/L and at heating up to 110 °C for several hours. Several steps of the acid hydrolysis in different media can be combined to achieve a better effect. Obtained hydrolyzates are neutralized, diluted and analyzed by appropriate separation method (usually liquid chromatography). Alternatively, monosaccharide mixtures can undergo reduction and then acetylation or silylation to obtain volatile derivatives (alditol acetates or methylsilanes) for gas chromatography (GC). Pure glucans should consist in only one monosaccharide, i.e., glucose. Therefore, total hydrolysis of these polysaccharides reveals glucose only, and detection of other sugars confirms impurity of glucan preparations.
The methylation analysis (MA) commonly using in sugar linkage determination in glucans includes following steps (74):
(I) Methylation of all hydroxyls in the polysaccharide. Disappearing of a broad absorption band at 3,200-3,700 cm–1 (OH stretching) in FTIR spectrum of methylated glucan confirms complete methylation;
(II) Cleavage of the methylated glucan into the monosaccharidic fragments. This treatment leads to braking of all glycosidic linkages, but not methylether ones. Strong acidic hydrolysis, methanolysis or acetolysis are suitable for such degradation;
(III) Reduction of formed aldehydes followed by acetylation or silylation. This step of MA leads to partially methylated alditols (or silans), which are acetylated (silylated) at the former linkage positions. Acetolysis at the previous step leads to complete acetylation of free hydroxyls;
(IV) Separation analysis of the alditol derivatives. Equipped with flame ionization (FID) or mass spectrometric (MS) detectors, GC is commonly used for this purpose. Positions of glycosidic linkages in the parent glucan correspond to non-methylated hydroxyls in methylated alditols (silans), while all free hydroxyls should be methylated. Structures of O-methylglucitols formed from different Glcp units of β-D-glucans are summarized in Table 3.
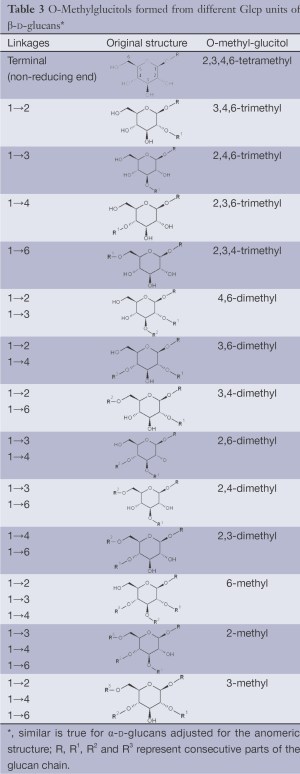
Full table
Selective methods of cleavage (reductive cleavage, periodate oxidation, Smith degradation, enzymatic digestion, etc.) are alternative to classical MA. These methods are also used in structural analysis of glucans.
Reductive cleavage using a combination of TMS-mesylate and BF3/Et2O avoids the formation of meso-alditols during MA and thus prevents the loss of structural information (75). In this case the final products are methylated at the anomeric position and acetylated at non-reducing linkage positions. The time consuming hydrolytic step is avoided by this approach. Reductive cleavage is effective for structural characterization of branched glucans with acid-labile groups or residues (76). In addition, it is possible to distinguish pyranose and furanose forms of the same sugar. Reductive cleavage is known to be specific for some glycosidic bonds. For example, only (1→4)-α-linkages in pullulan were effectively cleaved during MA, while the (1→6)-α-linkages remained intact (77).
Periodate oxidation of glucans leads to specific cleavage of C-C linkages between vicinal hydroxyls in Glcp units and formation of two aldehyde groups on the end carbons of the opening. When two neighbor C-C bonds are cleaved, formic acid is formed from the intermediate carbon atom. It is evident that periodate oxidation is impossible for the internal Glcp units in the (1→3)-linked sequence having no hydroxyls at vicinal position; only end units can be cleaved. This is true for both (1→3)-α and (1→3)-β-glucans. Contribution of the end glucoses is negligible for glucans with a long resistant backbone, so an amount of periodate used for oxidation is proportional to the DB. Sites of periodate attacks on different Glcp units in β-glucans are shown in Table 4. Smith degradation includes periodate oxidation, reduction of new aldehyde groups and hydrolysis of acetals. This approach is useful in structural analysis of branched glucans. Only in the case of (1→3)-linked backbone Smith degradation gives a resistant linear polysaccharide that could be analyzed by 13C NMR or other method; otherwise more deep destruction occurs. The structure of backbone and side chains is deduced from the spectra of parent and Smith-degraded glucans. For example, Smith-degraded derivative of botryosphaeran, a polysaccharide from ascomyceteous fungus Botryosphaeria sp., was defined as linear (1→3)-β-D-glucan; the side chains of this polysaccharide are single Glcp or (1→3)-β-linked glucooligosaccharides (35). Similarly, cell wall polysaccharide from yeast Schizosaccharomyces pombe was identified as a highly (1→3)-β-branched (1→6)-β-D-glucan (78). The size and distribution of repeating blocks of resistant units can be estimated in mixed linkage glucans.
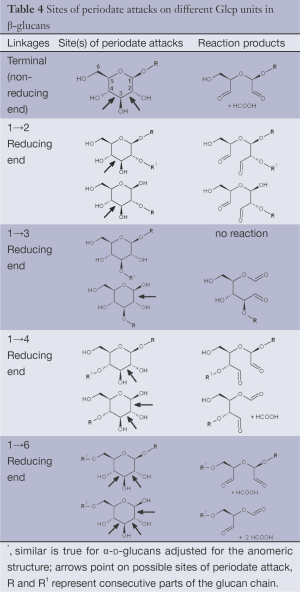
Full table
Mild acid hydrolysis and enzymatic cleavage of specific glycosidic bonds have been also used in structural analysis of mixed linkage or branched glucans. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry was used to analyze oligosaccharides released by mild acid hydrolysis of (1→3)(1→6)-β-D-glucan isolated from fruiting bodies of Ganoderma lucidum, dextran and curdlan (79). These glucans were also studied by MA combined with tandem mass (MS/MS) spectrometry. Cellulosic oligosaccharide released by lichenase cereal (1→3)(1→4)-β-D-glucan and lichenan were analyzed by HPLC (80). It was found that the cereal glucans were similar but had different ratios of (1→3)-β-linked cellotriosyl and cellotetraosyl fragments (both constituted about 90% of these polysaccharides). By contrast, lichenan was defined mainly as a polymer of (1→3)-β-linked cellotriosyls.
Vibration spectroscopy
FTIR spectroscopy is a powerful tool of the structural analysis of polysaccharides (81,82). This method is sensitive to the position and anomeric configuration of glycosidic linkages in glucans. It is possible to analyze glucans in crude high molecular fractions isolated from various raw materials. For example, fruiting bodies of mushrooms contain two main types of glucans: branched (1→3)(1→6)-β-D-glucan and linear (1→3)-α-D-glucan containing. Other compounds, mainly proteins, can be also present in the isolated fractions. FTIR spectra of high molecular fractions isolated from fruiting bodies of oyster mushroom Pleurotus ostreatus demonstrate characteristic bands of both glucans and proteins (Figure 1), and the relationship between these bands indicates the protein/glucan ratio. Two spectral regions are important for structural characterization of polysaccharides. These are “sugar region” (1,200-950 cm–1) and “anomeric region” (950-750 cm–1). The highly overlapping intense bands of CO and CC stretching vibrations in glycosidic bonds and pyranoid ring predominate in the former region. The latter region contains weak bands of complex skeletal vibrations sensitive to anomeric structure of glucose.
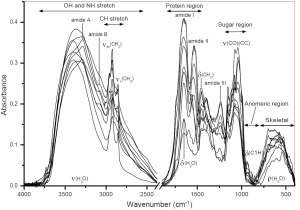
FTIR was used for analysis of A and B starch granules (83). The bands at 3,405 and 2,930 cm–1 were assigned to OH and CH bond stretching, respectively. The next bands at 1,420 and 1,366 cm–1 arose from bending modes of CH2, CH and OH. Intense overlapped bands in the region of 800-1,300 cm–1 correspond to CO, CC stretching and COH bending modes; several bands below 800 cm–1 correspond to ring and skeletal modes (84). Partially, the bands at 1,047 and 1,022 cm–1 are associated with the ordered and amorphous structures in starch, respectively (85). The ratio of these bands was used to quantify the degree of order in starch samples (86). However, the former band is composed of two overlapping components at 1,040 and 1,053 cm–1; both are sensitive to starch retrogradation, but in different manner (87).
FTIR spectrum of cellulose is sensitive to crystalline and amorphous state of this polysaccharide (88). The shift of the CH stretching band from 2,900 cm–1 to higher wavenumbers as well as significant lowering of this band indicates the presence of amorphous cellulose. In addition, the loss of crystallinity also leads to decrease or disappearing of some bands in the region of 900-1,500 cm–1. Partially, the band of CH2 scissoring at 1,430 cm–1 is known as the marker of crystallinity. Fall of its intensity indicates reduction in the crystallinity degree. By contrast, the IR band at 898 cm–1 assigned to COC stretching of glycosidic bonds is the amorphous state marker; its intensity increases for amorphous cellulose. Celluloses were also analyzed by the second derivative (SD) FTIR (89) as well as by the use of dynamic mechanical analysis (DMA) and 2D step-scan FTIR spectroscopy (90). Greater resolution of SD FTIR exhibited differences in several IR bands assigned mainly to vibrations of groups involved in the system of hydrogen bonds in cellulose (89). Partially, the broad band at 3,100-3,700 cm–1 (OH stretching) possesses information about the intra- and intermolecular hydrogen bonds. Cellulose sheets were stretched sinusoidally at low strains while being irradiated with polarized infrared light (90). For the obtained dynamic IR signals (in-phase and out-of-phase responses), the dynamic IR cross-correlation was defined. It consisted of two terms referred to synchronous and asynchronous 2D FTIR correlation intensities. As a result, the mentioned region can be resolved giving several distinct bands for inter- and intramolecular hydrogen bonds.
FTIR spectroscopy has been used to distinguish various fungal glucans in polysaccharidic fractions isolated from fruiting bodies of mushrooms and from other fungal sources (45,91). It was found that IR bands near 1,160, 1,078, 1,044 and 890 cm–1 are characteristic for fungal (1→3)(1→6)-β-D-glucans, while the bands of (1→4)(1→6)-α-D-glucans are located near 1,155, 1,023, 930, 850 and 765 cm–1. The band intensity ratios at 1,160/1,155, 1,041/1,023, 1,160/1,023, and 889/930, 889/765 cm–1, respectively, can be used to estimate the relationship between α- and β-linkages in glucan preparations (91). Alkali soluble fungal (1→3)-α-D-glucan can be detected by characteristic IR bands near 1,366, 930, 850, 822, 542, 454 and 420 cm–1 (45,92-95).
Raman spectroscopy has been applied in structural analysis of plant, animal and microbial glucans-starch, glycogen, cellulose, dextran, etc(96-100). The substitution at C-4 position of α-D-glucans leads to the appearance of an intense Raman band at 470-485 cm–1 (amylose, amylopectin) instead of that at 540-545 cm–1 (dextran) (100). In the case of pullulan no strong bands were found at these regions. Raman spectroscopy was used for the determination of amylose content in starch (101), study of starch gel retrogradation (102,103) and identification of modified starches according to their origin and type of modification (104). Structural differences in celluloses originated from various sources (plant and bacterial cell walls) were analyzed by Raman and FTIR spectroscopy (105). Both methods were suitable for cellulose structure assessment in terms of crystallinity and allomorphic structure. Various Raman bands were used for determination of crystallinity, while FTIR spectra were sensitive to structural changes in cellulose caused by non-cellulosic polysaccharides (pectin, xylan). Two methods (uni- and multivariate ones) based on FT Raman spectroscopy were developed to determine cellulose crystallinity (106). Raman crystallinity index (Xc Raman) was created to characterize the degree of crystallinity of partially crystalline cellulose I samples (107). This value was based on the crystallinity dependence of CH2 bending modes. Relative intensity ratios of the Raman lines at 1,481 and I 1,462 cm–1 correlated linearly with the substitution of crystalline cellulose I in calibration mixtures. Obtained Xc Raman values of microcrystalline cellulose of different origin were in agreement with the corresponding values obtained from NMR spectroscopy (Xc NMR).
FT-Raman and NMR spectroscopy were useful in analysis of cereal and fungal β-D-glucans. These methods are able to detect α-glucan impurities and predict configuration of β-linkages in cereal (1→3)(1→4)-β-D-glucan preparations (108,109). Both these methods were used in structural characterization of yeast (1→3)(1→6)-β-D-glucan films (110). The Raman band at 423 cm–1 is indicative for (1→3)-β-D-glucan, whereas three bands at 948, 864 and 554 cm–1 are characteristic to (1→3)-α-D-glucan (45).
The backscattered Raman optical activity (ROA) spectra of two glucans, pullulan and laminarin, were analyzed in comparison with the spectra of their oligomers-maltotriose and laminaribiose (110). Several differences were found comparing the ROA spectra of laminarin and laminaribiose. Four ROA signals at 1,050-1,150 cm–1 and signal near 445 cm–1 had reversed signs, and ROA signal near 1,431 cm–1 was increased in intensity, respectively. The last two signals were assigned to vibrations involving the glycosidic link. Observed spectral differences were explained by triple helix formation in laminarin. In contrast, ROA spectrum of pullulan, which adopts a random coil conformation in aqueous solution, was very similar to that of maltotriose.
NMR spectroscopy
NMR spectroscopy has been effectively used in structural analysis of various glucans (1,45,110). This method is able to determine anomeric configuration of Glcp units and the way of glycosidic linking between them (111). Anomeric proton and carbon signals at 4.9-5.1 and 98-100 ppm, respectively, indicate α-D-glucans. Corresponding resonances at 4.3-4.6 and 103-104 ppm are characteristic for β-D-glucans. Downfield shift of a specific carbon signal by ~5-10 ppm in comparison with those of the corresponding anomer of 1-methylglucoside indicates direct participation of this carbon in the glycosidic bond. Therefore, these shifts permit to distinguish substituted and non-substituted carbons in Glcp units. In some cases the assignment of resonance signals is complicated owing to overlapping and it is difficult to assign proton and carbon signals of specific sugar residues. Two-dimensional correlation NMR techniques are able to help solving problems with interpretation. Following steps are often used in NMR analysis of complex glucans (111): (I) the assignment of CH2/CH proton signals in Glcp units (COSY and TOCSY experiments); (II) the assignment of corresponding carbon signals (HETCOR, HMQC and/or HSQC experiments); (III) analysis of inner and inter-unit interactions between nuclei (NOESY, ROESY and/or HMBC experiments); (IV) construction of the sequence model based on the signal assignments.
Complete assignment of the 1H and 13C signals of amylose was made using correlation NMR spectroscopy and deuterium exchange (112). The same approach together with an INEPT experiment was used to assign the resonance signals of linear and branched glucan fragments as well as of branching glucan units of amylopectin. Cellulose fragments (DP ~15) were analyzed by NMR in NaOD/D2O solutions (113). The proton and carbon resonances were studied at different NaOD concentrations to detect dissociation of the hydroxyls. All CH proton resonances were upfield shifted linearly with an increase in the NaOD concentration due to electron-shielding effect. The shifting patterns of carbon resonances varied among the six carbons: C1 and C4 carbons undergo the electron-shielding effect, whereas the other carbons demonstrated the electron-deshielding effect. NMR spectra also confirmed that among all hydroxyls C3-OH has the highest resistance to dissociation. High-resolution 13C NMR studies of cellulose and cellulose oligomers dissolved in the ionic liquid 1-butyl-3-methylimidazolium chloride showed that the (1→4)-β-linked glucose oligomers are disordered in this medium (114). Several (1→3)(1→6)-β-D-linked glucans (laminaran, curdlan, yeast glucan, scleroglucan, etc.) were analyzed by correlation NMR spectroscopy (115). The ratios between specific H1 signals were used for estimation of the DP and DB. The DP and DB of water-soluble laminaran were 33 and 0.07, respectively; the corresponding values of water-insoluble yeast glucan were 228 and 0.003. Curdlan was identified as linear (1→3)-β-D-glucan (DB =0), while the DB of scleroglucan was 0.33.
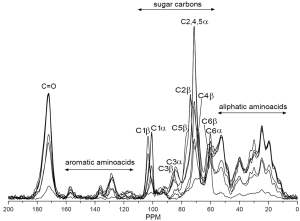
Solid state NMR techniques, like 13C cross polarization magic angle spinning (CP-MAS) NMR, possesses information about the structure of native celluloses (116) and cellulose polymorphs (117). Solid-state 13C NMR was used to characterize the molecular ordering of cellulose in a plant primary cell-wall preparation from the leaves of Arabidopsis thaliana (118). Proton and 13C spin relaxation time constants showed that the cellulose was in a crystalline state. Digital resolution enhancement revealed signals indicative of triclinic and monoclinic crystalline forms of cellulose. Proton rotating-frame relaxation experiments provided the clearest distinction between cellulose and other cell wall components. 13C CP/MAS NMR spectroscopy was used in structural analysis of gel formed by barley mixed-linked (1→3)(1→4)-β-D-glucan (119). Results showed that the polysaccharide chains contained regions of unique crystalline A type and amorphous ones. Authors concluded that junction zones occur in the glucan gel owing to the interaction of two β-glucan chains in the A-conformation. Solid state 13C CP-MAS NMR spectroscopy was used to identify polymorphic forms of (1→3)-β-D-glucan (120) and evaluate a hydration effect on its conformation (121). This method was also applied to structural analysis of glucan gels (122,123). 13C CP-MAS NMR was used for identification of glucan fractions isolated from oyster mushrooms (44). Branched (1→3),(1→6)-β-D-glucans were found as the main polysaccharide component predominated in hot water extracts partially as protein complexes, in alkali extracts together with (1→3)-α-D-glucans, and in alkali-insoluble solids as a complex with chitin. Like FTIR, 13C CP-MAS NMR is able to evaluate the ratio between protein and glucans as well as identify the type of glucan in the crude high molecular fractions (Figure 2) because the chemical shift of carbon signals are sensitive to the type of glycosidic bonds.
Conclusions
Analysis of glucan preparations needs assistance of various methods to estimate the purity, molecular weight and configuration of glucan macromolecules. These methods have some advantages and limitations, so cooperative using of different methods and approaches may bring more complete structural information about target glucans. In this context, combination of spectroscopic, chemical and separation techniques is especially effective for structural analysis of complex glucans.
Acknowledgements
This work was supported by the project No. CEZ: MSM6046137305 of the Ministry of Education of the Czech Republic.
Disclosure: The authors declare no conflict of interest.
References
- Synytsya A, Novák M. Structural diversity of fungal glucans. Carbohydr Polym 2013;92:792-809. [PubMed]
- Carbonero ER, Montai AV, Woranovicz-Barreira S, et al. Polysaccharides of lichenized fungi of three Cladina spp.: significance as chemotypes. Phytochemistry 2002;61:681-6. [PubMed]
- Thitipraphunkul K, Uttapap D, Piyachomkwan K, et al. A comparative study of edible canna (Canna edulis) starch from different cultivars. Part II. Molecular structure of amylose and amylopectin. Carbohydr Polym 2003;54:489-98.
- Luo X, Xu X, Yu M, et al. Characterisation and immunostimulatory activity of an α-(1→6)-d-glucan from the cultured Armillariella tabescens mycelia. Food Chem 2008;111:357-63.
- Han XQ, Wu XM, Chai XY, et al. Isolation, characterization and immunological activity of a polysaccharide from the fruit bodies of an edible mushroom, Sarcodon aspratus (Berk.) S. Ito. Food Res Int 2011;44:489-93.
- Painter TJ. Details of the fine structure of nigeran revealed by the kinetics of its oxidation by periodate. Carbohydr Res 1990;200:403-8.
- Bock K, Gagnaire D, Vignon M, et al. High resolution nuclear magnetic resonance studies of nigeran. Carbohydr Polym 1983;3:13-22.
- Tsumuraya Y, Misaki A, Takaya S, et al. A new fungal α-d-glucan, elsinan, elaborated by Elsinoe leucospila. Carbohydr Res 1978;66:53-65.
- Olafsdottir ES, Ingolfsdortir K, Barsett H, et al. Immunologically active (1-->3)-(1-->4)-alpha-D-glucan from Cetraria islandica. Phytomedicine 1999;6:33-9. [PubMed]
- Singh RS, Saini GK, Kennedy JF. Pullulan: microbial sources, production and applications. Carbohydr Polym 2008;73:515-31.
- Catley BJ, Whelan WJ. Observations on the structure of pullulan. Arch Biochem Biophys 1971;143:138-42. [PubMed]
- McIntyre DD, Vogel HJ. Structural studies of pullulan by nuclear magnetic resonance spectroscopy. Starch 1993;45:406-10.
- Reis RA, Tischer CA, Gorin PA, et al. A new pullulan and a branched (1→3)-, (1→6)-linked beta-glucan from the lichenised ascomycete Teloschistes flavicans. FEMS Microbiology Letters 2002;210:1-5. [PubMed]
- Sillerud LO, Shulman RG. Structure and metabolism of mammalian liver glycogen monitored by carbon-13 nuclear magnetic resonance. Biochemistry 1983;22:1087-94. [PubMed]
- Naessens M, Cerdobbel A, Soetaert W, et al. Leuconostoc dextransucrase and dextran: production, properties and applications. J Chem Technol Biotechnol 2005;80:845-60.
- Chen XY, Kim JY. Callose synthesis in higher plants. Plant Signal Behav 2009;4:489-92. [PubMed]
- Kiss JZ, Vasconcelos AC, Triemer RE. Structure of the euglenoid storage carbohydrate, paramylon. American J Botany 1987;74:877-82.
- Marchessault RH, Deslandes Y. Fine structure of (1→3)-β-D-glucans: curdlan and paramylon. Carbohydr Res 1979;75:231-42.
- McIntosh M, Stone BA, Stanisich VA. Curdlan and other bacterial (1-3)-β-D-glucans. Appl Microbiol Biotechnol 2005;68:163-73. [PubMed]
- Hoffmann GC, Simson BW, Timell TE. Structure and molecular size of pachyman. Carbohydr Res 1971;20:185-8. [PubMed]
- Read SM, Currie G, Bacic A. Analysis of the structural heterogeneity of laminarin by electrospray-ionisation-mass spectrometry. Carbohydr Res 1996;281:187-201. [PubMed]
- Kroon-Batenburg LM, Kroon J. The crystal and molecular structures of cellulose I and II. Glycoconj J 1997;14:677-90. [PubMed]
- Chawla PR, Bajaj IB, Survase SA, et al. Microbial cellulose: fermentative production and applications. Food Technol Biotechnol 2009;47:107-24.
- Pereyra MT, Prieto A, Bernabé M, et al. Studies of new polysaccharide from Lasallia pustulata (L.) Hoffm. Lichenologist 2003;35:177-85.
- Sassaki GL, Ferreira JC, Glienke-Blanco C, et al. Pustulan and branched β-galactofuranan from the phytopathogenic fungus Guignardia citricarpa, excreted from media containing glucose and sucrose. Carbohydr Polym 2002;48:385-9.
- Carbonero ER, Montai AV, Mellinger CG, et al. Glucans of lichenized fungi: Significance for taxonomy of the genera Parmotrema and Rimelia. Phytochemistry 2005;66:929-34. [PubMed]
- Liu Y, White PJ. Molecular weight and structure of water soluble (1→3),(1→4)-β-glucans affect pasting properties of oat flours. J Food Sci 2011;76:C68-74. [PubMed]
- Mandal S, Maity KK., Bhunia SK, et al. Chemical analysis of new water-soluble (1→6),(1→4)-α,β-glucan and water insoluble (1→3),(1→4)-β-glucan (Calocyban) from alkaline extract of an edible mushroom, Calocybe indica (Dudh Chattu). Carbohydr Res 2010;345:2657-63. [PubMed]
- Zhang Y, Li S, Wang X, et al. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocol 2011;25:196-206.
- Ohno N, Kurachi Y, Yadomae T. Physicochemical properties and antitumor activities of carboxymethylated derivatives of glucan from Sclerotinia sclerotiorum. Chem Pharm Bull (Tokyo) 1988;36:1016-25. [PubMed]
- Tada R, Adachi Y, Ishibashi K, et al. An unambiguous structural elucidation of a 1,3-β-D-glucan obtained from liquid-cultured Grifola frondosa by solution NMR experiments. Carbohydr Res 2009;344:400-4. [PubMed]
- Tabata K, Ito W, Kojima T, et al. Ultrasonic degradation of schizophyllan, an antitumor polysaccharide produced by Schizophyllum commune Fries. Carbohydr Res 1981;89:121-35. [PubMed]
- Coviello T, Palleschi A, Grassi M, et al. Scleroglucan: a versatile polysaccharide for modified drug delivery. Molecules 2005;10:6-33. [PubMed]
- Karácsonyi Š, Kuniak L. Polysaccharides of Pleurotus ostreatus: isolation and structure of pleuran, an alkali-insoluble β-d-glucan. Carbohydr Polym 1994;24:107-11.
- Barbosa AM, Steluti RM, Dekker RFH, et al. Structural characterization of botryosphaeran: a (1-3;1-6)-β-D-glucan produced by the ascomyceteous fungus, Botryosphaeria sp. Carbohydr Res 2003;338:1691-8. [PubMed]
- Chandra K, Ghosh K, Roy SK, et al. A water soluble glucan isolated from an edible mushroom Termitomyces microcarpus. Carbohydr Res 2007;342:2484-9. [PubMed]
- Chakraborty I, Mondal S, Pramanik M, et al. Structural investigation of a water-soluble glucan from an edible mushroom, Astraeus hygrometricus. Carbohydr Res 2004;339:2249-54. [PubMed]
- Olennikov DN, Agafonova SV, Rokhin AV, et al. Branched glucan from the fruiting bodies of Piptoporus betulinus (Bull.:Fr) Karst. Prikl Biokhim Mikrobiol 2012;48:74-80. [PubMed]
- Jarvis MC. Plant cell walls: supramolecular assemblies. Food Hydrocol 2011;25:257-62.
- Shi Z, Zhang Y, Phillips GO, et al. Utilization of bacterial cellulose in food. Food Hydrocol 2014;35:539-45.
- Lazaridou A, Biliaderis CG. Molecular aspects of cereal b-glucan functionality: physical properties, technological applications and physiological effects. J Cereal Sci 2007;46:101-18.
- Izydorczyk MS, Biliaderis CG. Structural and functional aspects of cereal arabinoxylans and β-glucans. In: Doxastakis G, Kiosseoglou V. eds. Novel Macromolecules in Food Systems. Amsterdam: Elsevier Science BV, 2000:361-84.
- Wasser SP. Medicinal mushrooms as a source of antitumour and immunomodulating polysaccharides. Appl Microbiol Biotechnol 2002;60:258-74. [PubMed]
- Stone BA, Clarke AE. Chemistry and biology of (1→3)-β-glucans. Melbourne, Australia: La Trobe University Press, 1992.
- An Synytsya. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: structure and potential prebiotic activity. Carbohydr Polym 2009;76:548-56.
- Jin Y, Zhang L, Tao Y, et al. Solution properties of water-insoluble (1-3)-α-D-glucan isolated from Poria cocos mycelia. Carbohydr Polym 2004;57:205-9.
- Zhang P, Zhang L, Cheng S. Effects of urea and sodium hydroxide on the molecular weight and conformation of α-(1-3)-D-glucan from Lentinus edodes in aqueous solution. Carbohydr Res 2000;327:431-8. [PubMed]
- Dubois M, Gilles KA, Hamilton JK, et al. Colorimetric method for determination of sugars and related substances. Anal Chem 1956;28:350-6.
- Dische S. Colour production and stability in the Folin and Wu method of blood glucose estimation. J Clin Pathol 1955;8:253-61. [PubMed]
- Smogyi M. Notes on sugar determination. J Biol Chem 1952;195:19-23. [PubMed]
- Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 1944;153:375-80.
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 1959;31:426-8.
- Lever M. A new reaction for colorimetric determination of carbohydrates. Anal Biochem 1972;47:273-9. [PubMed]
- Doner LW, Irwin PL. Assay of reducing end-groups in oligosaccharide homologues with 2,2'-bicinchoninate. Anal Biochem 1992;202:50-3. [PubMed]
- Martinez C. Determination of amylose in flour by a colorimetric assay: collaborative study. Starch 1996;48:86-9.
- Chen GC, Johnson BR. Improved colorimetric determination of cell wall chitin in wood decay fungi. Appl Environ Microbiol 1983;46:13-6. [PubMed]
- Ekblad A, Näsholm T. Determination of chitin in fungi and mycorrhizal roots by an improved HPLC analysis of glucosamine. Plant Soil 1996;178:29-35.
- Holan Z, Votruba J, Vlasáková V. New method of chitin determination based on deacetylation and gas-liquid chromatographic assay of liberated acetic acid. J Chromat 1980;190:67-76.
- Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem 1962;4:330-4. [PubMed]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem 1973;54:484-9. [PubMed]
- Downs F, Pigman W. Determination of O-acetyl groups by the Hestrin method. Methods Carbohydr Chem 1976;7:241-3.
- Jernejc K, Cimerma A, Perdih A. Comparison of different methods for protein determination in Aspergillus niger mycelium. Appl Microbiol Biotechnol 1986;23:445-8.
- Lynch JM, Barbano DM. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J AOAC Int 1999;82:1389-98. [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75. [PubMed]
- Bradford MM. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54. [PubMed]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965;16:144-58.
- You SG, Lim ST. Molecular characterization of corn starch using an aqueous HPSEC MALLS-RI system under various dissolution and analytical conditions. Cereal Chem 2000;77:303-8.
- Zhong F, Yokoyama W, Wang Q, et al. Rice starch, amylopectin, and amylose: molecular weight and solubility in dimethyl sulfoxide-based solvents. J Agric Food Chem 2006;54:2320-6. [PubMed]
- Cave RA, Seabrook SA, Gidley MJ, et al. Characterization of starch by size-exclusion chromatography: the limitations imposed by shear scission. Biomacromolecules 2009;10:2245-53. [PubMed]
- Blennow A, Mette Bay-Smidt A, Bauer R. Amylopectin aggregation as a function of starch phosphate content studied by size exclusion chromatography and on-line refractive index and light scattering. Int J Biol Macromol 2001;28:409-20. [PubMed]
- Striegel AM, Timpa JD. Gel permeation chromatography of polysaccharides using universal calibration. Int J Polym Anal Charact 1996;2:213-20.
- Zhang L, Ding Q, Zhang P, et al. Molecular weight and aggregation behaviour in solution of β-D-glucan from Poria cocos sclerotium. Carbohydr Res 1997;303:193-7.
- Zhang L, Ding Q, Meng D, et al. Investigation of molecular masses and aggregation of β-D-glucan from Poria cocos sclerotium by size-exclusion chromatography. J Chromatogr A 1999;839:49-55.
- Lindberg B, Lönngren J. Methylation analysis of complex carbohydrates: general procedure and application for sequence analysis. Methods Enzymol 1978;50:3-33. [PubMed]
- Rolf D, Gray GR. Reductive cleavage of glycosides. J Am Chem Soc 1982;104:3539-41.
- Methacanona P, Madla S, Kirtikara K, et al. Structural elucidation of bioactive fungi-derived polymers. Carbohydr Polym 2005;60:199-203.
- Rolf D, Bennek JR, Gray GR. Analysis of linkage positions in D-glucopyranosyl residues by the reductive-cleavage method. Carbohydr Res 1985;137:183-96.
- Sugawara T, Takahashi S, Osumic M, et al. Refinement of the structures of cell-wall glucans of Schizosaccharomyces pombe by chemical modification and NMR spectroscopy. Carbohydr Res 2004;339:2255-65. [PubMed]
- Hung WT, Wang SH, Chen CH, et al. Structure determination of β-glucans from Ganoderma lucidum with matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. Molecules 2008;13:1538-50. [PubMed]
- Wood PJ, Weisz J, Blackwell BA. Structural studies of (1→3)(1→4)-β-D-glucans by 13C-nuclear magnetic resonance spectroscopy and by rapid analysis of cellulose-like regions using high-performance anion-exchange chromatography of oligosaccharides released by lichenase. Cereal Chem 1991;71:301-7.
- Prado BM, Kim S, Özen BF, et al. Differentiation of carbohydrate gums and mixtures using Fourier transform infrared spectroscopy and chemometrics. J Agric Food Chem 2005;53:2823-9. [PubMed]
- Kačuráková M, Capek P, Sasinková V, et al. FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr Polym 2000;43:195-203.
- Zeng J, Li G, Gao H, et al. Comparison of A and B starch granules from three wheat varieties. Molecules 2011;16:10570-91. [PubMed]
- Cael JJ, Koenig JL, Blackwell J. Infrared and Raman spectroscopy of carbohydrates. Part IV: normal coordinate analysis of V-amylose. Biopolymers 1975;14:1885-903.
- Van Soest JJG, Tournois H, de Wit D, et al. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr Res 1995;279:201-14.
- Goni I, García-Diz L, Mañas E, et al. Analysis of resistant starch: a method for foods and food products. Food Chem 1996;56:445-9.
- Goodfellow BJ, Wilson RH. A Fourier transform IR study of the gelation of amylose and amylopectin. Biopolym 2004;30:1183-9.
- Ciolagu D, Ciolagu F, Popa VI. Amorphous cellulose – structure and characterisation. Cellulose Chem Technol 2011;45:13-21.
- Michell AJ. Second-derivative FTIR spectra of native celluloses from Valonia and tunicin. Carbohydr Res 1993;241:47-54.
- Hinterstoisser B, Salmén L. Application of dynamic 2D FTIR to cellulose. Vibrational Spectrosc 2000;22:111-8.
- Šandula J, Kogan G, Kačuráková M, et al. Microbial (1→3)-β-D-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohydr Polym 1999;38:247-53.
- Unursaikhan S, Xu X, Zeng F, et al. Antitumor activity of O-sulfonated derivatives of (1-3)-α-glucan from different Lentinus edodes. Biosci Biotechnol Biochem 2006;70:38-46. [PubMed]
- Wang T, Deng L, Li S, et al. Structural characterization of a water-insoluble (1-3)-α-D-glucan isolated from the Penicillium chrysogenum. Carbohydr Polym 2007;67:133-7.
- Zhang P, Zhang L, Chen S. Chemical structure and molecular weights of α-(1-3)-D-glucan. Bioscience Biotechnology Biochemistry 1999;63:1197-202.
- Chen J, Zhang L, Nakamura Y, et al. Viscosity behavior and chain conformation of a (1-3)-α-glucan from Ganoderma lucidum. Polym Bull 1998;41:471-8.
- Gałat A. Study of the Raman scattering and infrared absorption spectra of branched polysaccharides. Acta Biochim Pol 1980;27:135-42. [PubMed]
- Schenzel K, Fischer S. Application of Raman spectroscopy for the characterization of cellulose. Lenzinger Berichte 2004;83:64-70.
- Zhbankov RG, Firsov SP, Buslov DK, et al. Structural physico-chemistry of cellulose macromolecules. Vibrational spectra and structure of cellulose. J Mol Struct 2002;614:117-25.
- Santha N, Sudha KG, Vijayakumari KP, et al. Raman and infrared spectra of starch samples of sweet potato and cassava. Proc Indian Acad Sci 1990;102:705-12.
- Zhbankov RG, Firsov SP, Korolik EV, et al. Vibrational spectra and the structure of medical biopolymers. J Mol Struct 2000;555:85-96.
- Almeida MR, Alves RS, Nascimbem LB, et al. Determination of amylose content in starch using Raman spectroscopy and multivariate calibration analysis. Anal Bioanal Chem 2010;397:2693-701. [PubMed]
- Bulkin BJ, Kwak Y, Dea ICM. Retrogradation kinetics of waxy-corn and potato starches: a rapid, Raman-spectroscopic study. Carbohydr Res 1987;160:95-112.
- Fechner PM, Wartewig S, Kleinebudde P, et al. Studies of the retrogradation process for various starch gels using Raman spectroscopy. Carbohydr Res 2005;340:2563-8. [PubMed]
- Dupuy N, Laureyns J. Recognition of starches by Raman spectroscopy. Carbohydr Polym 2002;49:83-90.
- Szymańska-Chargot M, Cybulska J, Zdunek A. Sensing the structural differences in cellulose from apple and bacterial cell wall materials by raman and FT-IR spectroscopy. Sensors 2011;11:5543-60. [PubMed]
- Agarwal UP, Reiner RS, Ralph SA. Cellulose I crystallinity determination using FT-Raman spectroscopy: univariate and multivariate methods. Cellulose 2010;17:721-33.
- Schenzel K, Fischer S, Brendler E. New method for determining the degree of cellulose I crystallinity by means of FT Raman spectroscopy. Cellulose 2005;12:223-31.
- Mikkelsen MS, Jespersen BM, Larsen FH, et al. Molecular structure of large-scale extracted β-glucan from barley and oat: identification of a significantly changed block structure in a high β-glucan barley mutant. Food Chem 2013;136:130-8. [PubMed]
- Mikkelsen MS, Jespersen BM, Møller BL, et al. Comparative spectroscopic and rheological studies on crude and purified soluble barley and oat β-glucan preparations. Food Res Int 2010;43:2417-24.
- Bell AF, Hecht L, Barren LD. Polysaccharide vibrational Raman optical activity: laminarin and pullulan. J Raman Spectrosc 1995;26:1071-4.
- Mulloy B. High-field NMR as a technique for the determination of polysaccharide structures. Mol Biotechnol 1996;6:241-65. [PubMed]
- Falk H, Stanek M. Two-dimensional 1H and 13C NMR spectroscopy and the structural aspects of amylose and amylopectin. Monatshefte für Chemie 1997;128:777-84.
- Isogai A. NMR analysis of cellulose dissolved in aqueous NaOH solutions. Cellulose 1997;4:99-107.
- Moulthrop JS, Swatloski RP, Moyna G, et al. High-resolution 13C NMR studies of cellulose and cellulose oligomers in ionic liquid solutions. Chem Commun (Camb) 2005;1557-9. [PubMed]
- Kim YT, Kim EH, Cheong C, et al. Structural characterization of β-D-(1→3, 1→6)-linked glucans using NMR spectroscopy. Carbohydr Res 2000;328:331-41. [PubMed]
- Atalla RH, Van der Hart DL. The role of solid state 13C NMR spectroscopy in studies of the nature of native celluloses. Solid State Nucl Magn Reson 1999;15:1-19. [PubMed]
- Isogai A, Usuda M, Kato T, et al. Solid-state CP/MAS carbon-13 NMR study of cellulose polymorphs. Macromolecules 1989;22:3168-72.
- Newman RH, Davies LM, Harris PJ. Solid-state 13C nuclear magnetic resonance characterization of cellulose in the cell walls of Arabidopsis thaliana leaves. Plant Physiol 1996;111:475-85. [PubMed]
- Morgan KR, Roberts CJ, Tendler SJB, et al. A 13C CP/MAS NMR spectroscopy and AFM study of the structure of Glucagel™, a gelling β-glucan from barley. Carbohydr Res 1999;315:169-79.
- Pelosi L, Bulone V, Heux L. Polymorphism of curdlan and (1-3)-β-D-glucans synthesized in vitro: a 13C CP-MAS and X-ray diffraction analysis. Carbohydr Polym 2006;66:199-207.
- Fyfe CA, Stephenson PJ, Taylor MG, et al. Hydration effects in the carbon-13 CP/MAS NMR spectra of solid (1-3)-β-D-glucans. Macromol 1984;17:501-2.
- Saitô H, Ohki T, Sasaki T. A 13C-nuclear magnetic resonance study of polysaccharide gels. Molecular architecture in the gels consisting of fungal, branched (1-3)-β-D-glucans (lentinan and schizophyllan) as manifested by conformational changes induced by sodium hydroxide. Carbohydr Res 1979;74:227-40.
- Saitô H, Yoshioka Y, Yokoi M, et al. Distinct gelation mechanism between linear and branched (1-3)-β-D-glucans as revealed by high-resolution solid-state 13C NMR. Biopolymers 1990;29:1689-98. [PubMed]


