
Association between neurological deterioration and outcomes in patients with stroke
Introduction
Stroke is a disease characterized by high levels of disability and mortality worldwide (1). In China, approximately two million people suffer from strokes each year (2). The consequences of stroke-related disability and death are significant on both the individual and societal levels, and the deterioration of neurological function after stroke exacerbates the adverse outcomes. Therefore, the study of neurological deterioration (ND) may help to improve stroke prognosis.
In the neurology department at a tertiary hospital in China, the average length of stay for cerebral infarctions is seven to ten days, and some patients develop ND during hospitalization. Many scholars have studied ND, especially in the early stages (generally considered to be the first 48–72 hours after stroke onset), and have concluded that ND has a negative impact on prognosis (3-5). However, some patients with ND show improvements in neurological function by the time they are discharged from the hospital. Thus, it is likely that patients who show improvements in ND will have a different stroke prognosis than those who do not.
In this study, we focused on the group who did not show improvements in ND, and investigated the effects of the deterioration on short-term and long-term outcomes. Concurrently, we also explored whether ND in this group occurred during early or late hospitalization, and whether the time of occurrence affected the prognosis.
Methods
Patient selection
We retrospectively reviewed patient data from the stroke registry of the Department of Neurology of Tianjin Huanhu Hospital between October 1, 2008, and December 31, 2015. Diagnoses of acute cerebral infarction were made according to the World Health Organization criteria (6). The inclusion criteria were as follows: (I) age ≥18 years, (II) diagnosis of acute ischemic stroke (confirmed by computed tomography or magnetic resonance imaging), and (III) first-ever ischemic stroke having occurred within 7 days prior to admission. The exclusion criteria were as follows: (I) diagnosis of transient ischemic attack, cerebral hemorrhage, subarachnoid hemorrhage, brain tumors, or unspecified stroke; (II) history of serious medical disease(s), such as cancer, liver and/or kidney failure, cardiac insufficiency, or Parkinson’s disease; and (III) patients unwilling to participate.
The present study was approved by the Ethics Committee of Tianjin Huanhu Hospital. Informed consent was obtained from all individuals prior to inclusion in the study. This study can provide guidance for the prevention and treatment of ND in patients and help them improve their prognosis, and conforms to the Helsinki Declaration as revised in 2013.
Between 2008 and 2015, a total of 9,650 patients were analyzed at discharge after stroke. Within three months after stroke, four patients could not be followed up due to tumor, trauma, or other diseases. Additionally, 142 patients died from unrelated causes, and we were unable to get in touch with 87 patients for follow-up. Therefore, 9,417 patients were reviewed and analyzed. By the time of the 1-year follow-up, an additional nine patients could not be followed up due to other reasons (e.g., tumor, trauma, other diseases), 94 patients died from unrelated causes, and we were unable to get in touch with 445 patients. Therefore, 8,869 patients completed the follow-up 12 months after stroke onset (Figure 1).
Demographic data and clinical characteristics
We collected demographic and clinical data including stroke risk factors (hypertension, diabetes mellitus, atrial fibrillation, dyslipidemia, cerebral artery stenosis, obesity, smoking, and alcohol consumption), stroke subtypes, and blood test results (including triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and fasting glucose). Demographic data included sex and age.
Neurological assessment and definition of ND
The severity of neurological function deterioration was assessed by trained neurologists using the National Institutes of Health Stroke Scale (NIHSS) at admission and discharge. In this study, we defined ND as an increase in the NIHSS score by ≥4 points during hospitalization that did not resolve by discharge (NIHSS at discharge minus NIHSS at admission ≥4). Early neurological deterioration (END) was defined as an increase in the NIHSS score by ≥4 points between the baseline and 48-hour evaluations. Late neurological deterioration (LND) was defined as an increase in the NIHSS score by ≥4 points between the 48-hour and discharge evaluations.
Follow-up and outcome assessment
The patients were followed up for 1 year and were evaluated via in-person interviews 3 and 12 months post-stroke. The outcome data collected included functional impairment, recurrent cerebral infarction, intracranial hemorrhage, myocardial infarction, and death due to any cause. We used the mRS to evaluate functional impairment: Favorable outcome was defined as mRS score 0–2, and poor outcome as an mRS score ≥3, indicating that the patient could not live independently. The outcome endpoints were classified as either primary or secondary. The primary endpoint included two groups of patients: mRS 0–2 and 3–5; the secondary endpoint included three groups of patients, as follows: mRS 0–2, mRS 3–6, and a third group of patients with cerebral hemorrhage, recurrent stroke, and/or myocardial infarction.
Statistical analyses
Categorical variables were reported as proportions and compared using the chi-squared test, while continuous variables were reported as medians and interquartile ranges, and compared using the Kruskal-Walls H test and the Mann-Whitney U test. The relationships between ND and outcomes were evaluated using multivariate logistic regression, accounting for confounding variables that were identified as significant (P<0.05) in the univariate analysis. Two-tailed tests of significance were performed, and P values <0.05 were considered statistically significant. All statistical analyses were performed using the SPSS software package, version 17.0.
Results
Baseline demographics and clinical characteristics of patients with and without ND
The baseline demographic and clinical characteristics of patients with and without ND are presented in Table 1. A total of 9,650 patients [6,543 men (67.8%); median age, 65.3 years] were included in the present study. ND occurred in 293 patients during hospitalization. Among them, 192 (65.5%) were in the END group, and 101 (34.5%) were in the LND group. END and LND were associated with age, hypertension, diabetes, atrial fibrillation, and NIHSS at admission. There were no statistically significant differences with regard to smoking, drinking, obesity, dyslipidemia, atrial fibrillation, arteriosclerosis, or blood pressure on admission (Table 1).
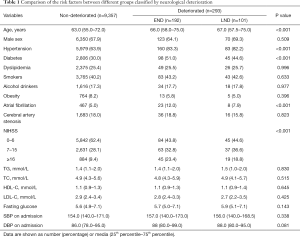
Full table
Risk factors between different groups classified by outcomes
Tables 2 and 3 show the comparison of the risk factors associated to the outcomes in the END and LND groups, respectively. In the END group, the risk factors associated with outcomes at the 3-month follow-up included age, male sex, END, and NIHSS score at admission. Similar results were obtained at the 1-year follow-up. The secondary endpoints at the 1-year follow-up were associated with diabetes and alcohol consumption. In the LND group, the results were similar to those of the END patients, as reported in Table 2. Specifically, at the 3-month follow-up, the outcomes were significantly associated with age, male sex, LND, and NIHSS score at admission. In addition, the secondary endpoints were significantly associated with cerebral artery stenosis. By the 1-year follow-up, the outcomes were significantly associated with age, male sex, LND, and NIHSS score at admission. Further, the secondary endpoints were also associated with diabetes and alcohol consumption. There were no significant differences between outcomes related to atrial fibrillation, hypertension, or fasting glucose, at either 3 months or 1 year.
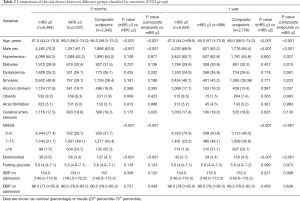
Full table
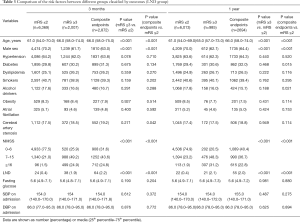
Full table
Association between ND and outcomes
Figure 2 show the multivariate logistic regression analyses of the ND groups and outcome variables. END and LND were significantly associated with poor outcomes at 3 months and 1 year. In the END group, ND was an independent predictor of poor primary and secondary outcomes at 3 months [model 1: odds ratio (OR) =8.069, 95% confidence interval (CI): 5.152–12.638, P<0.001; model 2: OR =8.194, 95% CI: 5.511–12.184, P<0.001) and one year (model 3: OR =7.895, 95% CI: 4.630–13.460, P<0.001; model 4: OR =5.679, 95% CI: 3.871–8.332, P<0.001). LND showed the same pattern of results at 3 months (model 5: OR =7.608, 95% CI: 4.283–13.515, P<0.001; model 6: OR =6.349, 95% CI: 3.758–10.727, P<0.001) and one year (model 7: OR =10.793, 95% CI: 5.428–21.459, P<0.001; model 8: OR =5.245, 95% CI: 3.057–9.000, P<0.001).
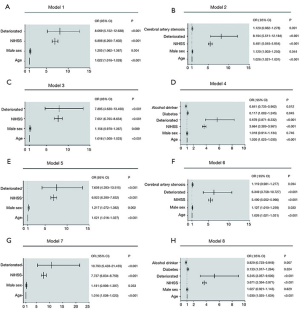
In addition, age, male sex, and NIHSS score at admission were associated with outcomes in the END (model 1, model 2) and LND groups (model 5, model 6) at the 3-month follow-up after adjusting for confounding variables. At the 1-year follow-up, age and NIHSS scores at admission were independent predictors of primary and secondary outcomes in the END (model 3, model 4) and LND groups (model 5, model 6) after adjusting for confounding variables. Moreover, models 4 and 8 showed that diabetes and alcohol consumption were independent predictors of secondary endpoint outcomes at the 1-year follow-up in both the END and LND groups.
Discussion
Our study found that the development of ND during hospitalization was associated with poor short- and long-term outcomes. Importantly, the outcomes depended on whether ND occurred in the early or late phase of hospitalization.
Previous studies have focused on the factors affecting ND, such as old age (7,8), systolic blood pressure at admission (9,10), diabetes mellitus (11,12), stroke severity (13,14), and C-reactive protein (15), among others. In our study, we found that age, hypertension, diabetes, NIHSS score at admission, and atrial fibrillation (16) were independent predictors of ND (Table 4). Several hypotheses have been proposed regarding the mechanisms of ND, including excitotoxicity, inflammation, oxidative stress, and cortical spreading depression (17). To date, few studies have analyzed the relationship between ND during hospitalization and prognosis (18). Kim et al. showed that functional status at discharge is strongly associated with long-term mortality (19). Further, recurrent strokes have been found to be associated with mortality (20), and cerebral and myocardial infarctions may be reciprocal in nature, leading to poor prognosis (21). Many studies have focused on the deterioration of neurological symptoms occurring in the first few days after stroke; however, some patients develop ND after this timeframe. Ma et al. focused on the latter patient group, and found that watershed infarcts and middle cerebral artery (MCA) and basilar artery (BA) stenosis or occlusion were independent risk factors for ND, as was pneumonia (18). However, the study did not analyze the relationship between ND and prognosis in this group of patients. In addition, some studies have examined ND occurring between 7 and 10 days after admission, but focused more so on the factors affecting ND, rather than on the effects of ND on prognosis, and in particular long-term prognosis (8,22). Davalos et al. divided patients into the early progressing stroke (EPS) and late progressing stroke (LPS) groups based on a 24-hour cutoff, and found that LPS was a high-risk factor for poor prognosis at 90 days (8), which is consistent with our findings. Appelros et al. found that stroke severity was significantly associated with mortality and dependency one year after stroke onset (23), which has been confirmed in other studies (24,25). Stroke severity was also a predictor of recurrent stroke, as found in many other studies (26), and is consistent with our results. In addition, Kim et al. showed that patients who experienced ND while in the hospital due to acute ischemic stroke had a higher risk of short- and long-term mortality, irrespective of initial stroke severity (19). We also confirmed that patients who experienced ND during hospitalization and had not yet recovered at discharge had poor short- and long-term outcomes. Notably, our study confirmed that ND occurring in the later period of hospitalization was also associated with poor outcomes at 3 months and 1 year.
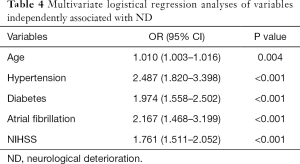
Full table
We found that LND predicted adverse outcomes at 3 months, including functional disability, cerebral hemorrhage, stroke recurrence, and myocardial infarction. Similar results were seen at the 1-year follow-up. Thus, the effect of ND on prognosis in patients with stroke is independent of the time at which ND occurs. Specifically, ND that occurs during hospitalization and does not resolve by discharge is predictive of a poor prognosis. We also found that ND during hospitalization might be associated with the recurrence of stroke, cerebral hemorrhage, or myocardial infarction, which is consistent with previous research (27).
It is worth noting that our research demonstrated that ND was associated with poor outcomes regardless of whether it occurred in the early or late period of hospitalization. We divided ND as occurring either early or late during hospitalization using a 48-hour cutoff point, and found that both END and LND were independent predictors of poor short- and long-term outcomes. Previous studies have mainly focused on the short-term (3 months) prognosis of END, and confirmed the strong association of END with poor functional outcomes, including mortality and dependency (13,28-31), which is consistent with our results. However, few studies have examined long-term prognosis, with the longest follow-up period for the effect of END on prognosis being 18 months (4). The authors found that END in patients with acute ischemic stroke was an independent predictor of poor outcomes after 18 months.
The present study has some limitations. First, the single-center design may have resulted in selection bias. Second, we defined END as an NIHSS score ≥4 points, thus ignoring some patients whose NIHSS score had changed by less than 4 points. Indeed, this group of patients also had ND, thus, further research is needed to properly evaluate this group.
Despite these limitations, our findings have important clinical implications. Specifically, we found that the development of ND during hospitalization was an independent predictor of poor short- and long-term outcomes, regardless of whether ND occurred in the early or late stages. This suggests that the prevention of ND during hospitalization can help improve patient outcomes. In addition, we found that age, sex, NIHSS score at admission, diabetes, and drinking were independent predictors of ND. Therefore, hypoglycemia and abstinence may help to prevent the occurrence of ND. Together, these findings can help predict patient outcomes and guide treatment strategies in patients with stroke.
Conclusions
We showed that ND during hospitalization was an independent predictor of poor short- and long-term outcomes. Moreover, the same conclusion was reached regardless of whether ND occurred in the early or late stages of hospitalization.
Acknowledgments
We would like to thank Editage for their English language editing services.
Funding: This work was supported by the National Natural Science Foundation of China (grant number 81671169, to J Wu); and the Tianjin Municipal Science and Technology Commission (grant number 17JCZDJC36500, to J Wu).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was approved by the Ethics Committee of Tianjin Huanhu Hospital. Informed consent was obtained from all individual participants prior to inclusion in the study. The study conformed to the Helsinki Declaration as revised in 2013, available at: http://www.wma.net/en/30publications/10policies/b3/%20index.html. This study can provide guidance for the prevention and treatment of ND in patients and help them improve their prognosis.
References
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010;121:948-54. [Crossref] [PubMed]
- Liu L, Wang D, Wong KS, et al. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke 2011;42:3651-4. [Crossref] [PubMed]
- Zinkstok SM, Beenen LF, Majoie CB, et al. Early deterioration after thrombolysis plus aspirin in acute stroke: a post hoc analysis of the Antiplatelet Therapy in Combination with Recombinant t-PA Thrombolysis in Ischemic Stroke trial. Stroke 2014;45:3080-2. [Crossref] [PubMed]
- Geng HH, Wang Q, Li B, et al. Early neurological deterioration during the acute phase as a predictor of long-term outcome after first-ever ischemic stroke. Medicine. 2017;96:e9068. [Crossref] [PubMed]
- Zhang YB, Su YY, He YB, et al. Early Neurological Deterioration after Recanalization Treatment in Patients with Acute Ischemic Stroke: A Retrospective Study. Chin Med J (Engl) 2018;131:137-43. [Crossref] [PubMed]
- Schlegel D, Kolb SJ, Luciano JM, et al. Utility of the NIH Stroke Scale as a predictor of hospital disposition. Stroke 2003;34:134-7. [Crossref] [PubMed]
- Toni D, Fiorelli M, Bastianello S, et al. Acute ischemic strokes improving during the first 48 hours of onset: predictability, outcome, and possible mechanisms. A comparison with early deteriorating strokes. Stroke 1997;28:10-4. [Crossref] [PubMed]
- Dávalos A, Toni D, Iweins F, et al. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke. 1999;30:2631-6. [Crossref] [PubMed]
- Dávalos A, Cendra E, Teruel J, et al. Deteriorating ischemic stroke: risk factors and prognosis. Neurology 1990;40:1865-9. [Crossref] [PubMed]
- Roquer J, Rodriguez-Campello A, Gomis M, et al. Acute stroke unit care and early neurological deterioration in ischemic stroke. J Neurol 2008;255:1012-7. [Crossref] [PubMed]
- Weimar C, Mieck T, Buchthal J, et al. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol 2005;62:393-7. [Crossref] [PubMed]
- Barber M, Wright F, Stott DJ, et al. Predictors of early neurological deterioration after ischaemic stroke: a case-control study. Gerontology 2004;50:102-9. [Crossref] [PubMed]
- Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. QJM 2006;99:625-33. [Crossref] [PubMed]
- Tei H, Uchiyama S, Ohara K, et al. Deteriorating ischemic stroke in 4 clinical categories classified by the Oxfordshire Community Stroke Project. Stroke 2000;31:2049-54. [Crossref] [PubMed]
- Seo WK, Seok HY, Kim JH, et al. C-reactive protein is a predictor of early neurologic deterioration in acute ischemic stroke. J Stroke Cerebrovasc Dis 2012;21:181-6. [Crossref] [PubMed]
- Prencipe M, Culasso F, Rasura M, et al. Long-term prognosis after a minor stroke: 10-year mortality and major stroke recurrence rates in a hospital-based cohort. Stroke 1998;29:126-32. [Crossref] [PubMed]
- Saia V, Pantoni L. Progressive stroke in pontine infarction. Acta Neurol Scand 2009;120:213-5. [Crossref] [PubMed]
- Ma Y, Liu L, Pu Y, et al. Predictors of neurological deterioration during hospitalization: results from the Chinese Intracranial Atherosclerosis (CICAS) Study. Neurol Res 2015;37:385-90. [Crossref] [PubMed]
- Kim YD, Song D, Kim EH, et al. Long-term mortality according to the characteristics of early neurological deterioration in ischemic stroke patients. Yonsei Med J 2014;55:669-75. [Crossref] [PubMed]
- Brandler ES, Sharma M. Early dual antiplatelet therapy in stroke: should we take the CHANCE? Ann Transl Med 2015;3:177. [PubMed]
- Akinseye OA, Shahreyar M, Heckle MR, et al. Simultaneous acute cardio-cerebral infarction: is there a consensus for management? Ann Transl Med 2018;6:7. [Crossref] [PubMed]
- Wang Y, Hu S, Ren L, et al. Lp-PLA2 as a risk factor of early neurological deterioration in acute ischemic stroke with TOAST type of large arterial atherosclerosis. Neurol Res 2019;41:1-8. [Crossref] [PubMed]
- Appelros P, Nydevik I, Viitanen M. Poor outcome after first-ever stroke: predictors for death, dependency, and recurrent stroke within the first year. Stroke 2003;34:122-6. [Crossref] [PubMed]
- Petty GW, Brown RD Jr, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke 2000;31:1062-8. [Crossref] [PubMed]
- Anderson CS, Jamrozik KD, Broadhurst RJ, et al. Predicting survival for 1 year among different subtypes of stroke. Results from the Perth Community Stroke Study. Stroke 1994;25:1935-44. [Crossref] [PubMed]
- Sacco RL, Foulkes MA, Mohr JP, et al. Determinants of early recurrence of cerebral infarction. The Stroke Data Bank. Stroke 1989;20:983-9. [Crossref] [PubMed]
- Vemmos KN, Bots ML, Tsibouris PK, et al. Prognosis of stroke in the south of Greece: 1 year mortality, functional outcome and its determinants: the Arcadia Stroke Registry. J Neurol Neurosurg Psychiatry 2000;69:595-600. [Crossref] [PubMed]
- Birschel P, Ellul J, Barer D. Progressing stroke: towards an internationally agreed definition. Cerebrovasc Dis 2004;17:242-52. [Crossref] [PubMed]
- Castillo J. Deteriorating stroke: diagnostic criteria, predictors, mechanisms and treatment. Cerebrovasc Dis 1999;9 Suppl 3:1-8. [Crossref] [PubMed]
- Jørgensen HS, Reith J, Nakayama H, et al. Potentially reversible factors during the very acute phase of stroke and their impact on the prognosis: is there a large therapeutic potential to be explored? Cerebrovasc Dis 2001;11:207-11. [Crossref] [PubMed]
- Helleberg BH, Ellekjaer H, Rohweder G, et al. Mechanisms, predictors and clinical impact of early neurological deterioration: the protocol of the Trondheim early neurological deterioration study. BMC Neurol 2014;14:201. [Crossref] [PubMed]



