
Restoration of FKBP51 protein promotes the progression of castration resistant prostate cancer
Introduction
Defining the etiology of castration-resistant prostate cancer (CRPC) remains a field of active investigation, while focuses on the androgen receptor (AR) signaling are still growing in the androgen independent stage of prostate cancer (PCa). Efficacy of novel hormonal therapies, Abiraterone and Enzalutamide, supports that AR signaling continues to be the primary molecular pathway driver in CRPC patients (1-3). While effective AR-target therapies can’t make PCa curable, due to intrinsic and acquired resistance to first line androgen deprivation therapies (ADTs) and other hormonal therapies. Molecular mechanisms of resistance are largely driven by AR aberrations including AR gene amplification, AR protein overexpression, AR gene mutations, and AR variants (AR-Vs) (4). All these AR aberrations activates AR signaling continuously in CRPC. Importantly, AR-V7 containing the AR DNA-binding domain (DBD) and the AR transcriptional activation domain, capable of transcriptional regulation, has been studied a lot owing to its clinical utility as a potential marker for CRPC treatment (5-7).
The FK506 binding protein FKBP51 belongs to the family of immunophilins (8), which has androgen induced RNA and protein expression in LNCaP cells as a target gene of AR-FL (9). FKBP51 has also been suggested to be induced by AR-V7, a truncated AR protein lacking the AR ligand-binding domain (LBD), to activate AR signaling (10). It’s well known that FKBP51 gene is frequently overexpressed in PCa (11). The C-terminal of FKBP51, three-unit domain of tetratricopeptide repeat (TPR) motifs, are involved in interaction with many proteins like Hsp90 (12,13). The N-terminal region of FKBP51 includes two FKBP-like domains: FK1, encompasses the PPIase function; FK2, lacks measurable PPIase activity (14,15). FKBP51 has been identified as a scaffolding protein that can enhance PH domain leucine-rich repeat protein phosphatase (PHLPP)-AKT interaction and facilitate PHLPP-mediated dephosphorylation of AKT-Ser473 (16). While, restoration of FKBP51 expression also contributes to NF-κB signaling upregulation (17,18) through IKKα activating, leading to subsequent phosphorylation of IκB and Ser 536 of P65 (18,19). In present study, we suggest that restoration of FKBP51, transcriptionally promoted by AR-V7, contributes to progression of PCa in androgen-absent condition.
Methods
Tissue specimens
CRPC specimens used in this study were surgical specimens and acquired by transurethral resection from PCa patients with complete clinicopathological data. These samples (n=11) were paraffin-embedded and subjected to immunohistochemistry analysis with standard DAB staining protocols. All studies were approved by the Ethics Committee of the Second Hospital of Tianjin Medical University, and informed consent was obtained from all patients.
Cell culture, cell lines, and transfection
The parental androgen dependent human prostate cancer cell line LNCaP was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). This cell line was maintained in RPMI-1640 medium (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco), 100 ng/mL streptomycin and 100 U/mL penicillin (Gibco). For androgen deprivation, parental LNCaP cells were maintained in RPMI 1640 medium supplemented with 10% charcoal-stripped fetal bovine serum (BI, Cromwell, CT, USA). The LNCaP-AI cell line was generated by long-term culturing of the parental LNCaP cells under androgen deprived conditions. Cells were incubated at 37 °C with 5% CO2. Transfection of LNCaP-AI cells reaching 50–70% confluency with shRNA was performed using lentivirus (GENECHEM, Shanghai China), according to the manufacturers’ instructions. Target sequences for FKBP51, AR-V7 and AR-FL shRNA used in this study were ACCTAATGCTGAGCT, GTAGTTGTGAGTATCATG, TCAAGGAACTCGATCGTAT, respectively. FKBP51, AR-V7 and AR-FL overexpressed lentivirus were purchased from GENECHEM (Shanghai, China).
RT-PCR analysis
The expression of FKBP51 mRNA in terms of LNCaP-AI generation was detected with RT-PCR. Total RNA was isolated from cells using Trizol reagent (Invitrogen, Waltham, MA, USA). The first strand cDNA synthesis was completed with the Reverse Transcription System (Roche, Indianapolis, IN, USA) following the manufacturer’s protocol. GAPDH was used as a loading control. Primer sequences: GAPDH, forward 5'-GGAGCGAGATCCCTCCAAAAT-3' and reverse 5'-GGCTGTTGTCATACTTCTCATGG-3', FKBP51, forward 5'-ATGAAGAAAGCCCCACAGC-3' and reverse 5'-CCTCACCATTCCCCACTCT-3'.
Antibodies
The following antibodies were used for immunofluorescence, immunoblotting and immunohistochemistry: AR-FL (Abcam,ab74272, 1:1,000 dilution for immunoblotting, 1:200 dilution for immunohistochemistry), AR-V7 (RevMAb Biosciences, 31-1109-00, 1:1,000 dilution for immunoblotting, 1:500 dilution for immunohistochemistry), FKBP51 (Abcam, Cambridge, UK, ab46002, 1:250 dilution for immunoblotting, 1:200 dilution for immunohistochemistry), NF-κB (Cell Signaling Technology, #8242, 1:1,000 dilution for immunoblotting, 1:800 dilution for immunohistochemistry), p-NF-κB (phosphor-S536) (Abcam, ab86299, 1:5,000 dilution for immunoblotting, 1:1,000 dilution for immunohistochemistry, 1:100 dilution for immunofluorescence), c-Myc (Abcam, ab56, 1:500 dilution for immunohistochemistry, 1:1,000 dilution for immunoblotting).
Immunoblot analysis
LNCaP lineage cells were washed with PBS and lysed in RIPA buffer (50 mM Tris-HCl/pH 7.4; 1% NP-40; 150 mM NaCl; 1 mM EDTA; 1 mM proteinase inhibitor; 1 mM Na3VO4; 1 mM NaF; 1 mM okadaic acid; and 1 mg·mL−1 aprotinin, leupeptin, and pepstatin). Protein samples were separated on 10% SDS-PAGE gel and transferred to PVDF membranes at 4 °C (250 mA, 2 h). The membranes were developed in ECL mixture and visualized by Imager.
Immunohistochemistry (IHC)
FFPE sections of 5 µm thickness were prepared on charged glass slides. After deparaffinization and rehydration, slides were immersed in 10 mM citrate buffer (PH =7.5) and microwaved at 750 W for 30 minutes for antigen retrieval. Endogenous peroxidase activity was blocked by adding 3% hydrogen peroxide. The sections were incubated with diluted antibodies followed by polymer conjugated horseradish peroxidase in a humidified chamber. Standard DAB staining was performed for chromogenic detection of the IHC targets.
Cell growth assay
Cell growth was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-nyltetrazolium bromide proliferation assays. Each experiment was performed in triplicate and repeated at least three times.
Immunofluorescence staining (IF)
Cells were grown on cover glasses and fixed with 4% paraformaldehyde in PBS for 10 minutes, After permeabilization with 0.2% Triton X-100 for 10 minutes and incubation with blocking buffer (PBS with 5% BSA) for 20 minutes, the cells were incubated with p-NF-κB (phospho S536) (Abcam, ab86299) overnight at 4 °C, and then with donkey anti-rabbit antibody at room temperature for 2 hours. Cell nucleus was stained with DAPI. The stained cover glasses were mounted on standard slides and examined under Olympus FV1000D microscope.
TUNEL assays
Cells were fixed with formaldehyde and incubated in 70% ethanol for 30 min at 4 °C. The staining process was performed according to the TUNEL assays protocol (Abcam). Then the images were received from confocal microscope.
Luciferase reporter assays
Primers with restriction enzyme sites HindIII/NheI were used to amplify the promoter ofFKBP51 from genomic DNA.DNA fragments were cloned into pGL4.27 promoter luciferase vector (Promega, Beijing, China). COS-1 cells were transfected with the FKBP51 promoter luciferase reporter constructs with AR-FL or AR-V7 overexpressing plasmid (Gene Copoeia, Guangzhou, China), separately. These cells were also treated with double hydrogen testosterone (DHT, 10 nM) for 48 hours. Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) was applied to measure luciferase value according to the manufacturer’s instructions.
DNA pull down
Promoter ofFKBP51 from genomic DNA was amplified via RT-PCR, then labeled with biotin. LNCaP, LNCaP-P30 and LNCaP-AI cells nuclear extracts were prepared and binding assays were performed as described in Wu’s paper (20).
Bioinformatics analysis
RNA-seq data from shAR-V7 and shAR-FL mediated knock-down experiments in LN95 cell lines was received from GEO database (GSE106560). Fold change of FKBP51 expression in response to DHT stimulation was presented.
Statistical analysis
Summary data were expressed as mean ± SD. The Student’s t-tests were used to compare to the experimental groups. P value of <0.05 (two-sided) was considered to indicate a statistically significant difference.
Results
Restoration of FKBP51 promotes the growth of PCa cells after androgen deprivation
FKBP51 has been reported to be induced by androgen in LNCaP cells and be upregulated in metastatic androgen independent PCa compared to localized prostate tumors and normal prostate tissues (9). Mulholland et al. suggested that conditional deletion of AR-FL in epithelium downregulates androgen-responsive gene FKBP51 to promote the proliferation of Pten-null PCa, leading to CRPC progression (21). To investigate biological function of FKBP51 in CRPC progression, we generated an androgen-independent LNCaP-AI cell line by long-term culturing of androgen-dependent LNCaP cells in RPMI-1640 medium containing charcoal-stripped serum, which has been described in our previous study (17). This LNCaP-AI cell line was used to mimic the castration resistant condition after PCa treatment. During the establishment of LNCaP-AI, we found that mRNA and protein level of FKBP51 decreased first and then increased (Figure 1A,B). To evaluate the function of FKBP51 in the restoration process, FKBP51 was overexpressed in androgen-absent cultured passage 30 LNCaP cells (LNCaP-P30) with lentivirus transfection. The efficiency of FKBP51 overexpression was confirmed in Figure 1C by western blot. Then, MTT assays were used to determine the cells growth. The survival curves indicated growth of LNCaP-P30 cells were promoted by FKBP51 overexpression (Figure 1C). Knockdown of FKBP51 in LNCaP-AI cells induced the suppression of growth. The efficiency of knockdown was determined by western blot (Figure 1D). Bouwmeester et al. found that RNAi of FKBP51 blocked activation of NF-κB probably through inhibiting the interaction with IKKα (18). We found alteration of p-NF-κB (Ser536) was similar with FKBP51 expression during the construction of LNCaP-AI cell line (17). Apoptosis of LNCaP-AI cells was valued to be enhanced after FKBP51 depletion through TUNEL assays (Figure 1E). Together, restoration of FKBP51 expression attenuated apoptosis of androgen independent PCa cells.
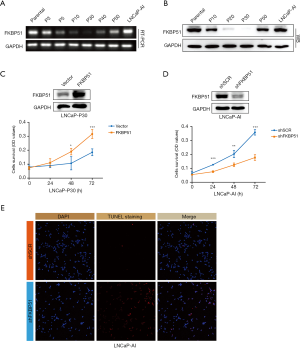
FKBP51 enhances activity of NF-κB signaling in the progression of CRPC
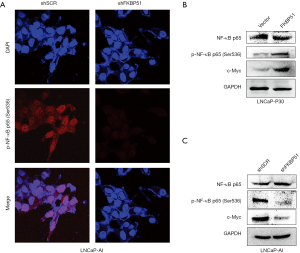
To confirm the impact of FKBP51 on NF-κB signaling activation in androgen ablation condition, we performed immunofluorescence assays in LNCaP-AI cells transfected with FKBP51 shRNA. The efficacy of FKBP51 knocked down was shown in Figure 1D. Level of p-NF-κB (Ser536) decreasing suggested inhibition of NF-κB signaling after FKBP51 knocked down in the absence of androgen (Figure 2A). NF-κB signaling and its downstream gene c-Myc were found to be upregulated in LNCaP-P30 cells with overexpressed FKBP51 (Figure 2B). Overexpression of FKBP51 was presented in Figure 1C. We also showed that level of p-NF-κB (Ser536) and c-Myc went down after FKBP51 knocked down in LNCaP-AI cells without influencing the expression of NF-κB (Figure 2C).
Transcriptional re-activation of FKBP51 depends on AR-V7, not AR-FL in CRPC
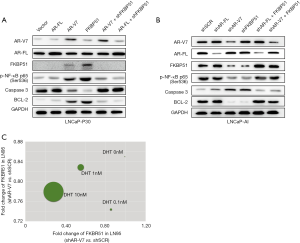
As AR-FL target gene, FKBP51 expression was suggested to be induced by androgen (9,22). However, in androgen-absent condition, it seems that FKBP51 may not be regulated by AR-FL. It is well known that androgen-receptor isoform encoded by splice variant 7 lacks the LBD which is the target of enzalutamide and abiraterone, and remains constitutively active as a transcription factor (23). We hypothesize that replacement of AR-FL by AR-V7 may promote the transcription of FKBP51 in androgen absent condition. Toward this end, we generated an androgen-independent, AR-V7 positive LNCaP-AI cell line by long-term culturing of androgen-dependent LNCaP cells in RPMI-1640 medium containing charcoal-stripped serum that mimics the castration-resistant condition (Figure 3A) (17).

Then, we overexpressed AR-FL and AR-V7 in LNCaP-P30 cells, respectively. AR-V7 overexpression induced FKBP51 expression in LNCaP-P30 cells, while AR-FL overexpression didn’t (Figure 3B). AR-FL and AR-V7 were knocked down in LNCaP-AI cells, respectively. Depletion of AR-V7 attenuated the level of FKBP51 protein, however, knockdown of AR-FL had little impact on FKBP51 (Figure 3C). FKBP51 promoter luciferase reporter was transfected into AR-FL or AR-V7 overexpressed COS1 cells. Cultured without DHT, AR-V7 enhanced the luciferase signal, not AR-FL (Figure 3D). In presence of DHT (10 nM), AR-FL tend to increase higher luciferase signal than that of AR-V7 (Figure 3E). DNA pull down assays were used to confirm the interaction between AR-V7 protein and biotin-labeled FKBP51 promoter probe. No binding of AR-V7 protein and FKBP51 promoter was detected in parental LNCaP cells (Figure 3F). AR-V7 proteins interacted with FKBP51 promoter probe in LNCaP-AI and LNCaP-P30 cells (Figure 3F). Results above suggested AR-V7 promoted FKBP51 gene expression as a transcriptional factor in absence of androgen.
AR-V7/FKBP51/NF-κB signaling axis promotes the progression of CRPC
To validate AR-V7/FKBP51/NF-κB signaling axis in absence of androgen, AR-FL, AR-V7 and FKBP51 were overexpressed in LNCaP-P30 cells, respectively. Increasing of AR-V7 and FKBP51expression induced the level of p-NF-κB (Ser536) and Bcl-2 while downregulated expression of caspase 3 (Figure 4A). However, overexpression of AR-FL had little impact on FKBP51 and NF-κB signaling. Then, we knocked down the expression of FKBP51 in AR-V7 or overexpressed AR-FL LNCaP-P30 cells, which attenuated the activity of NF-κB signaling (Figure 4A). Next, LNCaP-AI cells were transfected with AR-V7, AR-FL and FKBP51 shRNA respectively. The level of p-NF-κB (Ser536) was determined to be lower in LNCaP-AI shAR-V7 cells than that of LNCaP-AI cells transfected with shSCR. Overexpression of FKBP51 in LNCaP-AI cells with AR-V7 or AR-FL knocked down increased the activity of NF-κB signaling (Figure 4B) which is well known to be aberrantly activated in CRPC (24,25). We also used a recently published RNA-seq database to validate our results (26). The RNA-seq data was retrieved from GSE106560, and the expression of FKBP51 was analyzed after knockdown of AR-V7 or AR-FL in LN95 cells. In absence of DHT, knockdown of AR-V7 decreased the level of FKBP51, while AR-FL had little impact on FKBP51 in LN95 cells (Figure 4C). Treatment with DHT, AR-FL depletion tend to attenuate FKBP51 expression dramatically, however, knockdown of AR-V7didn’t perturb FKBP51 significantly (Figure 4C). All the results above indicated that AR-V7 activated FKBP51 transcriptionally, then increased NF-κB signaling in absence of androgen.
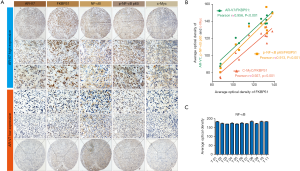
Level of AR-V7, FKBP51 and p-NF-κB are positively correlated in CRPC patients
To investigate the correlation of AR-V7, FKBP51 and NF-κB signaling in CRPC patients, we detected the level of AR-V7, FKBP51, NF-κB, p-NF-κB (Ser536) and c-Myc in eleven CRPC specimens by IHC assays. Two of the samples were presented in Figure 5A, one sample revealed a strong positive AR-V7 expression, the other showed a lower expression of AR-V7 than the strong positive one. We found that high expression of AR-V7 tend to be accompanied by higher expression of FKBP51, p-NF-κB (Ser536) and c-Myc in CRPC patients (Figure 5B). But, the level of NF-κB was similar among these patients (Figure 5C). The clinicopathological data also supports our hypothesis.
Discussion
PCa is the most commonly diagnosed malignancy among male and still ranks the third-leading cause of male cancer-related death in western countries (27). With development of magnetic resonance imaging (28,29) and prostate-specific antigen (PSA) screening (30,31), prostate cancer could be diagnosed at earlier stage. These patients are routinely treated with surgery, agents and radiation, and some of them are successfully cured (32,33). As it were, androgen signaling promotes the progression of prostate cancer (34). As such, ADT is recognized as a key treatment for patients with locally advanced, disseminated or biochemical recurrence prostate cancer. However, some patients initially sensitive to ADT will develop to be resistant to treatment, and progress into CRPC. Importantly, the AR signaling continues to be the primary driver in CRPC (1-3,35). AR-V7, one of AR variants lacking the AR ligand-binding domain (LBD), has been characterized to activate AR signaling as well as a potential clinical marker (5-7). In present study, we aim to identify the role of AR-V7/FKBP51/NF-κB signaling axis in the progression of CRPC.
Several studies have reported that FKBP51 gene is emerging as a potentially important downstream of androgen signaling in the prostate (36-39). Magee et al. established a direct in vivo link between AR-FL and a transcriptional enhancer located in FKBP5 gene, suggesting AR-FL as the transcriptional factor for FKBP51 (40). Our results are in agreement with previous studies. In our work, we found initial decreasing of FKBP51 expression in androgen depletion cultured LNCaP cells are because of inactivated AR-FL. However, recent studies have suggested that AR-V7 contains the AR-FL DBD and the AR-FL transcriptional activation domain, they are capable of transcriptional regulation, in spite of the loss of the AR-FL LBD (10,41). At the functional level, ADT induces increased expression of AR-V7 due to relief of androgen mediated inhibition of AR gene transcription (42). Lacking LBD does not make the function of AR-V7 be influenced by either first-line or novel hormonal therapies currently used in the clinic. In present study, our luciferase assays and transfection of PCa cells with plasmid assays indicated that FKBP51 proteins were regulated by AR-V7 in androgen-absent condition, instead of AR-FL. This mechanism of re-activating AR signaling in androgen ablation condition contributes to the progression of CRPC.
FK506 binding proteins (FKBPs) are multifunctional proteins that highly conserved across the species and abundantly expressed in the cell. Some evidence supports an essential role for FKBP51 in the control of NF-κB signaling (18,39-42). An interaction of FKBP51 with IKKα was firstly identified in a study mapping the protein interaction network of the TNFα/NF-κB pathway (18). It is well known that NF-κB signaling is aberrantly activated in prostate cancer. Gasparian et al. reported that androgen-independent cell lines, such as PC-3 and DU-145, constitutively expressed higher levels of NF-κB than androgen-dependent cell lines, such as LNCaP and normal human prostate epithelial cells (25). Romano et al. suggested that FKBP51 upregulated NF-κB signaling by serving as an IKK scaffold protein in melanoma (19). In our study, we found that NF-κB signal pathway was re-activated in androgen resistant LNCaP-AI cells. In LNCaP-AI generation process, similar level fluctuation of FKBP51 and p-NF-κB (Ser536) was detected (17). Overexpression or knockdown of FKBP51 in LNCaP P30 or LNCaP-AI cells respectively also confirmed that NF-κB signaling could be regulated by FKBP51 in androgen-absent condition. Because of highly expressed AR-V7 in LNCaP-AI cells, transcriptionally promoted FKBP51 activates NF-κB signaling. Activated NF-κB signaling inhibits the apoptosis of PCa cells based on our TUNEL assays results. Interestingly, level of NF-κB p65 can’t be influenced by FKBP51 expression suggesting activating NF-κB signaling by FKBP51 mainly through FKBP51-IKKα interaction enhanced phosphorylation of NF-κB. According to our previous data, restoration of FKBP51 combined with upregulation of lncRNA PCAT1 contributes re-activates NF-κB signaling in androgen resistant PCa cells (17).
To validate the correlation of AR-V7, FKBP51 and NF-κB signaling, we determined the level of AR-V7, FKBP51, p-NF-κB (Ser536) and NF-κB signaling downstream target gene (c-Myc) in eleven CRPC patients’ tissues by IHC assays. The results indicated that expression of AR-V7, FKBP51, p-NF-κB (Ser536) and c-Myc was positively correlated in CRPC patients, which is consistent with cell line data. While, there are still a few limitations in present study. The vitro biological data was collected from a single LNCaP lineage cell line and the patient sample size was not large enough, and thus, limitations occurred. Further studies involving larger scale of patients are still needed to evaluate the significant role of AR-V7/ FKBP51/ NF-κB signaling axis.
Conclusions
In this study, by using a castration-resistant PCa cell model LNCaP-AI, we confirmed FKBP51, induced by expression of AR-V7, play a key role in the development of CRPC, which was functional with regulating NF-κB signaling pathway, inhibiting apoptosis of prostate cancer cells and promoting CRPC occurrence in androgen-absent condition. In a word, FKBP51 may be a strong therapeutic target gene for CRPC, potentially.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (grants 81872100 and 81772756), Natural Science Foundation of Tianjin (17JCZDJC35300 and 18JCZDJC34800), and Postgraduate Innovation Fund of ‘13th Five-Year comprehensive investment’, Tianjin Medical University (YJSCX201810).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All studies were approved by the Ethics Committee of the Second Hospital of Tianjin Medical University (No. KY2018K027), and informed consent was obtained from all patients.
References
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-2005. [Crossref] [PubMed]
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138-48. [Crossref] [PubMed]
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-97. [Crossref] [PubMed]
- Karantanos T, Evans CP, Tombal B, et al. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol 2015;67:470-9. [Crossref] [PubMed]
- Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215-28. [Crossref] [PubMed]
- Lu C, Luo J. Decoding the androgen receptor splice variants. Transl Androl Urol 2013;2:178-86. [PubMed]
- Luo J. Development of AR-V7 as a putative treatment selection marker for metastatic castration-resistant prostate cancer. Asian J Androl 2016;18:580-5. [Crossref] [PubMed]
- Gaali S, Gopalakrishnan R, Wang Y, et al. The chemical biology of immunophilin ligands. Curr Med Chem 2011;18:5355-79. [Crossref] [PubMed]
- Febbo PG, Lowenberg M, Thorner AR, et al. Androgen mediated regulation and functional implications of fkbp51 expression in prostate cancer. J Urol 2005;173:1772-7. [Crossref] [PubMed]
- Krause WC, Shafi AA, Nakka M, et al. Androgen receptor and its splice variant, AR-V7, differentially regulate FOXA1 sensitive genes in LNCaP prostate cancer cells. Int J Biochem Cell Biol 2014;54:49-59. [Crossref] [PubMed]
- Jääskeläinen T, Makkonen H, Palvimo JJ. Steroid up-regulation of FKBP51 and its role in hormone signaling. Curr Opin Pharmacol 2011;11:326-31. [Crossref] [PubMed]
- Baughman G, Wiederrecht GJ, Campbell NF, et al. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol Cell Biol 1995;15:4395-402. [Crossref] [PubMed]
- Gallo LI, Lagadari M, Piwien-Pilipuk G, et al. The 90-kDa heat-shock protein (Hsp90)-binding immunophilin FKBP51 is a mitochondrial protein that translocates to the nucleus to protect cells against oxidative stress. J Biol Chem 2011;286:30152-60. [Crossref] [PubMed]
- Sinars CR, Cheung-Flynn J, Rimerman RA, et al. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci U S A 2003;100:868-73. [Crossref] [PubMed]
- Nair SC, Rimerman RA, Toran EJ, et al. Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor. Mol Cell Biol 1997;17:594-603. [Crossref] [PubMed]
- Pei H, Li L, Fridley BL, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell 2009;16:259-66. [Crossref] [PubMed]
- Shang Z, Yu J, Sun L, et al. LncRNA PCAT1 activates AKT and NF-κB signaling in castration-resistant prostate cancer by regulating the PHLPP/FKBP51/IKKα complex. Nucleic Acids Res 2019;47:4211-25. [Crossref] [PubMed]
- Bouwmeester T, Bauch A, Ruffner H, et al. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol 2004;6:97-105. [Crossref] [PubMed]
- Romano S, Xiao Y, Nakaya M, et al. FKBP51 employs both scaffold and isomerase functions to promote NF-kappaB activation in melanoma. Nucleic Acids Res 2015;43:6983-93. [Crossref] [PubMed]
- Wu KK. Analysis of protein-DNA binding by streptavidin-agarose pulldown. Methods Mol Biol 2006;338:281-90. [PubMed]
- Mulholland DJ, Tran LM, Li Y, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 2011;19:792-804. [Crossref] [PubMed]
- Makkonen H, Kauhanen M, Paakinaho V, et al. Long-range activation of FKBP51 transcription by the androgen receptor via distal intronic enhancers. Nucleic Acids Res 2009;37:4135-48. [Crossref] [PubMed]
- Luo J, Attard G, Balk SP, et al. Role of Androgen Receptor Variants in Prostate Cancer: Report from the 2017 Mission Androgen Receptor Variants Meeting. Eur Urol 2018;73:715-23. [Crossref] [PubMed]
- Chaturvedi MM, Sung B, Yadav VR, et al. NF-kappaB addiction and its role in cancer: 'one size does not fit all'. Oncogene 2011;30:1615-30. [Crossref] [PubMed]
- Gasparian AV, Yao YJ, Kowalczyk D, et al. The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J Cell Sci 2002;115:141-51. [PubMed]
- Cato L, de Tribolet-Hardy J, Lee I, et al. ARv7 Represses Tumor-Suppressor Genes in Castration-Resistant Prostate Cancer. Cancer Cell 2019;35:401-13.e6. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Rastinehad AR, Turkbey B, Salami SS, et al. Improving detection of clinically significant prostate cancer: magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J Urol 2014;191:1749-54. [Crossref] [PubMed]
- Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 2011;59:477-94. [Crossref] [PubMed]
- Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA 2014;311:1143-9. [Crossref] [PubMed]
- Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986-2005. J Natl Cancer Inst 2009;101:1325-9. [Crossref] [PubMed]
- Klotz L, Emberton M. Management of low risk prostate cancer-active surveillance and focal therapy. Nat Rev Clin Oncol 2014;11:324-34. [Crossref] [PubMed]
- McLeod DG. Success and failure of single-modality treatment for early prostate cancer. Rev Urol 2004;6 Suppl 2:S13-9. [PubMed]
- Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev 2010;24:1967-2000. [Crossref] [PubMed]
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424-33. [Crossref] [PubMed]
- Amler LC, Agus DB, LeDuc C, et al. Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22-R1. Cancer Res 2000;60:6134-41. [PubMed]
- Mousses S, Wagner U, Chen Y, et al. Failure of hormone therapy in prostate cancer involves systematic restoration of androgen responsive genes and activation of rapamycin sensitive signaling. Oncogene 2001;20:6718-23. [Crossref] [PubMed]
- Nelson PS, Clegg N, Arnold H, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci U S A 2002;99:11890-5. [Crossref] [PubMed]
- Velasco AM, Gillis KA, Li Y, et al. Identification and validation of novel androgen-regulated genes in prostate cancer. Endocrinology 2004;145:3913-24. [Crossref] [PubMed]
- Magee JA, Chang LW, Stormo GD, et al. Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology 2006;147:590-8. [Crossref] [PubMed]
- Antonarakis ES, Armstrong AJ, Dehm SM, et al. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis 2016;19:231-41. [Crossref] [PubMed]
- Cai C, He HH, Chen S, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell 2011;20:457-71. [Crossref] [PubMed]


