Checkpoint inhibitors and progressive multifocal leukoencephalopathy: friends of foes?
Progressive multifocal leukoencephalopathy (PML) has evolved in the last decade from a disorder of acquired immune deficiency syndrome (AIDS) patients into a rare but fatal complication of several life-saving therapeutics (ranging from rituximab in non-Hodgkin lymphomas to natalizumab in multiple sclerosis). PML is linked to opportunistic reactivation of the latent human JC polyomavirus (JCV), which leads to emergence of neurotropic strains causing brain demyelination.
Evidences supporting CD4+ T cells lymphopenia as the preponderant risk factor for PML include AIDS being the disease with the highest incidence of PML in the pre-highly active antiretroviral therapy (HAART) era, and occurrence of more than 15 cases of PML in males with idiopathic CD4+ T cells lymphopenia (ICL) (a very rare immune deficiency preserving B lymphocyte and immunoglobulin levels) (1). Accordingly, the incidence of HIV-related PML has decreased after adoption of HAART, from 14.8/1,000 to 0.8/1,000 in 1996 and 2011, respectively (2), and in an ICL patient treated with the T-cell lymphopoietic drug recombinant human IL-7 PML went into complete remission once CD4+ T lymphocyte counts returned to normal (3).
CD4+ T cells lymphopenia is the shared mechanism for many drugs causing PML. Rituximab and other anti-CD20 monoclonal antibodies, dimethyl fumarate and natalizumab are all known to cause CD4+ T lymphopenia (4). Carotenuto and coworkers demonstrated that CD4+/CD8+ ratio during natalizumab therapy is related to anti-JCV antibody index at baseline and even more after 12 and 24 months of therapy (5). No consensus treatment for PML exists yet, and the rarity of the disease causes most cases to be enrolled in N-of-1 clinical trials. As for any other virus-driven disease, treatments can be classified as direct or indirect, as summarized in Table 1. In PML patients JCV-specific CD8+ cytotoxic T lymphocytes express PD-1 more frequently than total CD8+ T lymphocytes, and that PD-1 receptor blockage boosts JCV-specific T-cell immune response in a subgroup of PML patients (6). The finding was not unexpected since PD-1 expression on T lymphocytes is rapidly induced after antigen exposure, following engagement between TCR and its cognate epitope-loaded MHC molecule in the draining lymph nodes (7). PD-1 RNA expression is detected in T lymphocytes just 2 hours after TCR-specific stimulation (8).

Full table
In March 2019, Hoang et al. at Washington University reported the first PML patient successfully treated with nivolumab (3 mg/kg every 2 weeks for 5 doses) (9). Then in the April 2019 issue of New England Journal of Medicine, three independent hospitals described treatment of 6 out of 10 PML cases (a case series of 8 from NIH and 2 accompanying case reports from Toulouse and Freiburg) with the checkpoint inhibitors (CPi) pembrolizumab (2 mg/kg every 4–6 weeks for up to 3 doses) (10,11) or nivolumab (12). They are both IgG4 molecules that are small enough to cross the blood–brain barrier. The patients had different underlying conditions, including non-Hodgkin lymphoma, ICL, and HIV infection.
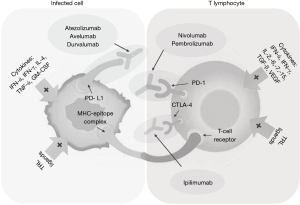
CPi (anti-PD-1 and anti-PD-L1) are extensively used nowadays as aspecific active immunotherapeutic agents that work by “awakening” senescent T lymphocytes in solid and hematological malignancies, hence restoring immune surveillance (Figure 1). Since PML is largely a disorder of immune deficiency, it makes sense that JCV immunity restoration improves PML course (13). Both pembrolizumab and nivolumab target PD-1, while ipilimumab targets CTLA-4, and atezolizumab, avelumab and durvalumab target PD-L1. Combination between anti-PD-1 and anti-PD-L1 has been shown synergic in anti-cancer therapies, and could be investigated in PML, too. Although the more than 70% success rate raised enthusiasms, several caveats apply:
- preexistence of JCV-specific CD4+ T cells was shown to be a prerequisite for therapeutic success, making ELISPOT a necessary screening before initiation of treatment;
- a decrease in the size of the lesions was interpreted as treatment efficacy, but in some cases, the PML lesions were replaced with atrophy, a finding consistent with destruction of white matter by PML rather than with elimination of the lesions. Furthermore, the absence of contrast enhancement on MRI suggests that the immune checkpoint blockers may not have been potent enough to trigger the immune reconstitution inflammatory syndrome (IRIS) (14);
- no data were disclosed regarding the effect of PD-1 blockade on JCV–specific CD8+ cytotoxic T lymphocytes, which are considered by some researchers the most important effectors of the cellular immune response (15);
- although the 11 patients had only minor side effects, severe side effects (such as autoimmune disorders or cytokine release syndrome) often accompany CPi-induced systemic immune activation. All these drugs have been approved by FDA since March 2015 on only, so their safety profile is still under investigation;
- two of the PML patients with HIV who experienced response to CPi also had therapy with HAART, although they were progressing before CPi treatment, and one PML patient with CLL had already achieved stable disease before initiating CPi treatment;
- these series did not include any organ transplant patients, and it would be interesting to see whether CPi work in this group without significant adverse events. CPi are relatively contraindicated in patients on steroids [such as those with autoimmune disorders and organ transplant, as steroids may (theoretically) prevent the immune stimulation] but have been used in transplant patients for treatment of malignancies, with both tumor regression and graft rejection (16). Hence, the risk to benefit ratio must be carefully considered before using CPi for PML in transplant patients (17);
- on the other side of the coin, 5 cases of PML have instead be reported after nivolumab (14,18) and 3 cases after pembrolizumab in EudraVigilance. So, it is not clear whether CPi are friends or foes for PML.
Sanjo et al. reported an increase in CD4+ T-cell infiltrations and a normal CD4:CD8 ratio in the PML good prognosis group. An increase in CD138+ plasma cells was also observed in the good prognosis group, and the number of CD138+ plasma cells was significantly associated with the number and PD-1+ cells). Regulatory plasma cells may regulate inflammatory T-lymphocytes activity by the PD-1/PD-L1 pathway, sparing uninfected brain from immune-mediated damage during ongoing JCV infection (19).
Educational management and risk minimization strategies should remain the main path to minimize PML incidence (20), but whenever it occurs, the therapeutic armamentarium has now one more weapon. What is amazing is that the world of virology and the world of CPi’s are having more and more cross points: e.g., oncolytic viruses synergize with CPi’s (21), CPi can be delivered via viral vectors (22), and CPi have proven effective at treating virus-associated cancers (23). To date CPi have been mostly associated with serious infection (e.g., aspergillosis, tuberculosis, listeriosis, pneumocystis pneumonia, and cytomegalovirus) either from immune dysregulation, drug-induced neutropenia, or from immunosuppression linked to the management of immune-related adverse events (24), but treating opportunistic chronic viral infections with host-directed CPi therapy could be one more use (25). T-cell exhaustion is well known in chronic infections: accordingly, PD-1 is actually overexpressed in HIV, hepatitis B and/or C virus patients due to chronic antigenic stimulation. As always, fine-tuning the risk-benefit ratio between exaggerate immune activation and immune reconstitution will remain the main hurdle.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Nambirajan A, Suri V, Kataria V, et al. Progressive multifocal leukoencephalopathy in a 44-year old male with idiopathic CD4+ T-lymphocytopenia treated with mirtazapine and mefloquine. Neurol India 2017;65:1061-4. [Crossref] [PubMed]
- Casado JL, Corral I, García J, et al. Continued declining incidence and improved survival of progressive multifocal leukoencephalopathy in HIV/AIDS patients in the current era. Eur J Clin Microbiol Infect Dis 2014;33:179-87. [Crossref] [PubMed]
- Alstadhaug KB, Croughs T, Henriksen S, et al. Treatment of progressive multifocal leukoencephalopathy with interleukin 7. JAMA Neurol 2014;71:1030-5. [Crossref] [PubMed]
- Focosi D, Tuccori M, Maggi F. Progressive multifocal leukoencephalopathy and anti-CD20 monoclonal antibodies: what do we know after 20 years of rituximab. Rev Med Virol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Carotenuto A, Scalia G, Ausiello F, et al. CD4/CD8 ratio during natalizumab treatment in multiple sclerosis patients. J Neuroimmunol 2017;309:47-50. [Crossref] [PubMed]
- Tan CS, Bord E, Broge TA Jr, et al. Increased program cell death-1 expression on T lymphocytes of patients with progressive multifocal leukoencephalopathy. J Acquir Immune Defic Syndr 2012;60:244-8. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Chemnitz JM, Parry RV, Nichols KE, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004;173:945-54. [Crossref] [PubMed]
- Hoang E, Bartlett NL, Goyal MS, et al. Progressive multifocal leukoencephalopathy treated with nivolumab. J Neurovirol 2019;25:284-7. [Crossref] [PubMed]
- Cortese I, Muranski P, Enose-Akahata Y, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med 2019;380:1597-605. [Crossref] [PubMed]
- Rauer S, Marks R, Urbach H, et al. Treatment of progressive multifocal leukoencephalopathy with Pembrolizumab. N Engl J Med 2019;380:1676-7. [Crossref] [PubMed]
- Walter O, Treiner E, Bonneville F, et al. Treatment of Progressive Multifocal Leukoencephalopathy with Nivolumab. N Engl J Med 2019;380:1674-6. [Crossref] [PubMed]
- Hohlfeld R. Immune checkpoint blockade for treating progressive multifocal leukoencephalopathy. Lancet Neurol 2019;18:623-4. [Crossref] [PubMed]
- Koralnik IJ. Can immune checkpoint inhibitors keep JC virus in check? N Engl J Med 2019;380:1667-8. [Crossref] [PubMed]
- Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol 2011;85:7256-63. [Crossref] [PubMed]
- De Bruyn P, Van Gestel D, Ost P, et al. Immune checkpoint blockade for organ transplant patients with advanced cancer: how far can we go? Curr Opin Oncol 2019;31:54-64. [Crossref] [PubMed]
- Venniyoor A. Immune checkpoint inhibitors for progressive multifocal leukoencephalopathy. Saudi J Kidney Dis Transpl 2019;30:998-9. [Crossref] [PubMed]
- Martinot M, Ahle G, Petrosyan I, et al. Progressive multifocal leukoencephalopathy after treatment with Nivolumab. Emerg Infect Dis 2018;24:1594-6. [Crossref] [PubMed]
- Sanjo N, Nose Y, Shishido-Hara Y, et al. A controlled inflammation and a regulatory immune system are associated with more favorable prognosis of progressive multifocal leukoencephalopathy. J Neurol 2019;266:369-77. [Crossref] [PubMed]
- Vukusic S, Rollot F, Casey R, et al. Progressive multifocal leukoencephalopathy incidence and risk stratification among Natalizumab users in France. JAMA Neurol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Sivanandam V, LaRocca CJ, Chen NG, et al. Oncolytic viruses and immune checkpoint inhibition: the best of both worlds. Mol Ther Oncolytics 2019;13:93-106. [Crossref] [PubMed]
- Lamichhane P, Deshmukh R, Brown JA, et al. Novel delivery systems for checkpoint inhibitors. Medicines (Basel) 2019. [Crossref] [PubMed]
- Gao P, Lazare C, Cao C, et al. Immune checkpoint inhibitors in the treatment of virus-associated cancers. J Hematol Oncol 2019;12:58. [Crossref] [PubMed]
- Del Castillo M, Romero FA, Argüello E, et al. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis 2016;63:1490-3. [Crossref] [PubMed]
- Rao M, Valentini D, Dodoo E, et al. Anti-PD-1/PD-L1 therapy for infectious diseases: learning from the cancer paradigm. Int J Infect Dis 2017;56:221-8. [Crossref] [PubMed]