
Intensive management of severe acute pancreatitis
Introduction
Severe acute pancreatitis (SAP) is a common acute abdominal disease that causes multiple organ dysfunction. Therefore, its treatment should be conducted in accordance with the principles of emergency medicine. During the acute response phase, especially within 72 h of onset (from the emergence of abdominal pain), if patients receive prompt and reasonable treatment, then the incidence and complications of late infections could be very low, thereby significantly improving the survival rate. Despite the progress in understanding the exacerbation mechanism of SAP, it is still difficult to further cross the “three mountains” [reducing hospitalization time, decreasing medical expenses, and improving the cure rate (85–90%)] involved in the treatment of acute pancreatitis (AP) (1). Therefore, optimizing the application method of current conventional treatment is important for improving prognoses. Based on our basic research, clinical practice, and literature reports, treatment has been optimized to create the “intensive management plan,” which involves two types of management plans that are intrinsically relevant and consistent with each other during the acute response phase and infection phase.
Three principles of the intensive management plan
The first principle of the intensive management plan is time management (time from onset to medical intervention). Initiating and completing the necessary treatment within a prescribed period of time in accordance with the principles of emergency medicine is imperative in the intensive management plan. The intensive management plan requires initiation and/or completion of treatment within 72 h of onset. Treatment measures include diagnosis, etiology identification, fluid resuscitation, assessment of abdominal compartment syndrome (ACS), intestinal protection, enteral nutrition, mitigation and blocking of the systemic inflammatory response (SIRS), maintenance of organ function, and early use of antibiotics.
During the infection phase, when sepsis occurs, the following four steps must be completed within 48 h: identify the site of infection; select appropriate antibiotics; assess and maintain organ function status; and evaluate the timing and strategy of surgical intervention.
The second principle of the intensive management plan is coordination. All treatments, whether during the acute phase or infection phase, must comply with the three R (3R) principles: right strategies, right sequence, and right ward (2).
The third principle of the intensive management plan is setting and achieving a goal. It is necessary to evaluate whether the necessary treatments have had the expected efficacy and to conduct a periodic assessment of treatment efficacy. Assessments should be performed every 4 h within 72 h of onset during the acute phase, and then every day until day 14. During the infection phase, assessments should be performed every 12 h after infection.
Intensive management plan during the acute response phase
Diagnosis
Confirmed diagnosis of SAP
The 2013 Guidelines of the International Association of Pancreatology (IAP) indicated that AP can be diagnosed based on the appearance of two or more of the following: clinical symptoms (epigastric pain); laboratory results (the level of serum amylase or lipase is more than three-times the upper limit of the normal value); and/or imaging observations (CT, MRI, ultrasonography) (2). SAP can be diagnosed when persistent organ failure lasts more than 48 h (3). Here, organ failure was determined based on a sepsis-related organ failure assessment (SOFA) score more than 2 (assessed using the worst cardiovascular, renal, and lung measurements within 24 h) or if vasoactive drugs were necessary, serum creatinine was 171 µmol/L, and PaO2/FiO2 was 300 mmHg. Persistent organ failure refers to organ failure that lasts more than 48 h, and temporary organ failure refers to organ failure that lasts less than 48 h (3).
The diagnostic criteria for AP may lead to false-positive results. Therefore, we recommend adopting at least one of the two diagnostic methods in addition to radiology.
Differential diagnosis
It is necessary to exclude pseudo-AP. There are two types of pseudo-AP: one that involves extra-pancreatic activation of pancreatic enzymes, whereby pancreatic fluid enters the retroperitoneal cavity, and one that does not involve pancreatic enzyme activation (pancreatic diffuse edema caused by systemic poisoning or inflammation of organs surrounding the pancreas). Knowing the difference between the two types can prevent misdiagnoses and incorrect treatment.
Etiology and treatment
After a diagnosis of SAP is confirmed, it is mandatory to immediately identify the etiology to prevent persistent injury (Figure 1).
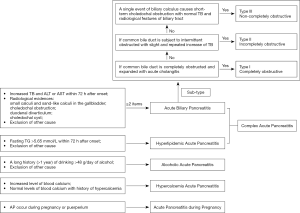
Acute biliary pancreatitis (ABP)
ABP is diagnosed based on the following: increased total bilirubin and/or transaminase (alanine aminotransferase and aspartate transaminase) level at any time within 72 h of onset; radiologic evidence of small calculi and sand-like calculi in the gallbladder, choledochal obstruction, duodenal diverticulum, or choledochal cyst; and exclusion of other causes. ABP can be diagnosed based on the simultaneous appearance of two or more of these indicators. However, ABP cannot be fully excluded based on normal liver functions.
Sub-types of ABP
Bile duct obstruction is divided into three types according to the state of the bile duct.
With the obstructive type (type I), the common bile duct is completely obstructed and concomitant with acute cholangitis.
With the incompletely obstructive type (type II), the common bile duct is free of obstruction or subject to repeated intermittent obstruction, is possibly concomitant with acute cholangitis, the level of total serum bilirubin is increased slightly or repeatedly, and the common bile duct may or may not expand.
With the non-obstructive type (type III), a single event of biliary calculus causes short-term choledochal obstruction, the level of total serum bilirubin does not increase, choledochectasia is absent, and there are no residual calculi in the gallbladder or common bile duct.
Treatment for ABP
Performing external biliary drainage in a timely manner not only can protect the function of multiple organs but also can eliminate etiological factors; therefore, it is a very important strategy (4,5). A widely accepted strategy is subjecting the patient to endoscopic retrograde cholangiopancreatography treatment within 48 h and an indwelling nasobiliary catheter as long as SAP is complicated with acute cholangitis (6). Therefore, for the obstructive and incompletely obstructive types of SAP, it is recommended that patients should undergo emergency endoscopic retrograde cholangiopancreatography/endoscopic nasobiliary drainage within 6 h after admission and no later than 48 h after onset. If endoscopic treatment fails or if treatment is unavailable, then emergency percutaneous transhepatic gallbladder drainage should be performed (7).
Hyperlipidemic pancreatitis
Hyperlipidemic pancreatitis can be diagnosed based on a history of hyperlipidemia and any fasting blood triglyceride levels >5.65 mmol/L within 72 h after onset (8). It is necessary to perform blood lipid adsorption, separation, or plasma exchange in emergency situations. Adsorption can be directly performed using polysulfone hemofilters (with replacement of the filters every 4 h). Separation and plasma exchange can be performed with a blood purifier to quickly reduce blood triglyceride levels (9).
Alcoholic AP
A long history (>1 year) of drinking >48 g/day of alcohol is used to diagnose alcoholic AP after other causes are excluded (10). There is no special treatment available, and its prognosis is poor.
AP during pregnancy
AP can occur during pregnancy or puerperium. In the case of severe pancreatitis, the pregnancy should be terminated and the etiologic factor should be resolved (11).
AP due to hypercalcemia
Patients with increased or normal levels of blood calcium and/or who have a history of hypercalcemia can develop AP. Calcitonin, bisphosphonate, and hemofiltration can reduce blood calcium levels (12).
Complex AP
The etiology of complex AP is consistent with the diagnostic criteria for ABP and hyperlipidemic pancreatitis. The etiologic factors should be resolved as soon as possible because this disease is more severe than any other form of AP.
Controlled fluid resuscitation
Indications
Controlled fluid resuscitation is only suitable for patients with severe blood volume deficiency within 72 h after onset and three or more of the following criteria: heart rate (HR) ≥120 beats/min; mean arterial pressure (MAP) ≥85 mm Hg or ≤60 mm Hg; blood lactate concentration (BLC) ≥2 mmol/L; urine output (UO) ≤0.5 mL·kg-1·h-1; and hematocrit (HCT) level ≥44%.
Strategies and methods for controlled fluid resuscitation
Blood volume expansion
At a crystal-to-colloid ratio of 2:1, infusion is performed using two vascular pathways simultaneously. If MAP is <60 mmHg, then it must be increased to >60 mmHg using a pressor agent and rapid infusion within 30 min, followed by controlling the optimum infusion rate (5–10 mL/kg/h). The total amount of infusion fluid should be controlled by evaluating it every 4 h, even if the blood volume expansion has reached the required standard. Blood volume expansion was considered to have reached the standard as long as it occurred slowly, within 24 h after admission, and when two or more of the following requirements were met: MAP 65 to 85 mmHg; normal urine volume; or HCT 30% to 35% (13,14).
Adjustment of body fluid distribution
After the blood volume expansion reached the required standard, the body fluid distribution should be quickly adjusted; excess liquid infused during the expansion phase should be excreted from the body or the continual loss of body fluid should be resuscitated (15). Infusion fluid is primarily composed of colloids and crystals (colloid-to-crystal ratio of 3:1) that have diuretics and/or continuous renal replacement therapies added to them.
Endpoint of fluid resuscitation
The gold standard for determining the endpoint of fluid resuscitation is the disappearance of oxygen debt. However, the determination of oxygen debt requires technology such as floating catheters or pulse contour cardiac output (16), which limit routine clinical applications. Therefore, it is imperative to use simple clinical indicators to determine the endpoint of liquid resuscitation. We propose that the disappearance of SIRS should be the endpoint of fluid resuscitation for SAP because the distance between capillaries becomes normal and oxygen intake returns to normal, so as to eliminate oxygen debt, only after the disappearance of SIRS; however, this view has not yet been widely accepted.
Intravenous bolus injection of large doses of vitamin C
Because vitamin C is an antioxidant that can reduce the total amount of crystal infusion (17), it is routinely injected intravenously at a high dose of 200 mg/kg/day for 3 to 7 days during the early stage of fluid resuscitation.
ACS
Intra-abdominal hypertension (IAH) that occurs during the acute response phase is mainly caused by rapid large-capacity expansion (liquid-type IAH) and severe abdominal flatulence due to retroperitoneal lesions (flatulence-type IAH). Intra-abdominal pressure (IAP) must be routinely monitored (every 6 h) within 72 h of onset.
Definition
IAH refers to IAP >12 mmHg, and ACS refers to IAP >20 mmHg with new visceral dysfunction or failure. Intervention should be initiated as soon as possible after IAH onset, and IAP should be controlled to <20 mmHg within 24 h (18).
Management principles
Five management measures are imperative for ACS: intestinal clearing; negative water balance; hemofiltration; surgical intervention; and analgesia and muscle relaxation (19). To resolve flatulence-type ACS, the first three of these five measures should be used. When IAP cannot be effectively reduced, it is necessary to add sedatives and muscle relaxants. If necessary, decompressive laparotomy should be performed (20); however, this is controversial. To resolve liquid-type ACS, the first four of the five measures should be used. When performing surgical intervention, the first-line treatment is the use of a percutaneous indwelling single-lumen catheter for passive drainage, followed by decompressive laparotomy if necessary (21).
Mitigation and blocking of SIRS
Basic measures
Timely removal of the etiological factors and fluid resuscitation are the most basic measures for mitigating and blocking SIRS.
Drugs
A continuous intravenous bolus injection of a broad-spectrum protease inhibitor (such as ulinastatin) at a dose of 1 million units/day until SIRS disappears is administered to resolve SIRS; this usually requires approximately 2 weeks (22). Another option is a continuous intravenous bolus injection of vitamin C at a high dose of 200 mg/kg/day at a rate of 1 to 2 g/h until the stress state disappears, and then the dose is reduced to 100 mg/kg/day, with the total application time usually lasting approximately 2 weeks (23). Attention should be focused on whether oxalate crystals are found in urine test results on a daily basis. If the test is positive for oxalate crystals, then vitamin C should be stopped. Chinese medicine is another treatment method for SIRS. Mirabilite, a traditional Chinese medicine, can be applied externally to the whole abdomen, up to the xiphoid process, down to the pubic symphysis, right to the right posterior axillary line, and left to the left posterior axillary line. In addition, Dachengqi decoction (100 mL bid) can be administered through a nasojejunal tube (24).
Hemofiltration
Hemofiltration can be started within 72 h of SIRS onset. For severe pancreatitis, intermittent venovenous hemofiltration (IVVH) is performed with a high-flow sodium bicarbonate substitution fluid. Stop if any of the following occur: heart rate ≤90/min or respiratory rate ≤20/min. IVVH should be suspended after 8 to 10 h. If SIRS continues to deteriorate after 12 to 24 h of observation, then IVVH needs to be initiated again (25). For fulminant pancreatitis, high-flow continuous venovenous hemofiltration should be used.
Percutaneous indwelling single-lumen catheter for passive drainage
Bloody exudate in the retroperitoneal cavity and abdominal cavity should be drained to reduce absorption (26).
Organ function support
Respiratory function
Patients with abnormal respiratory function (any of the following: dyspnea, respiratory rate including SaO2 95% or PaO2 5 to 10 kPa) and who still have respiratory function abnormality after 6 h of fluid resuscitation or treatment should undergo both invasive mechanical ventilation and high concentrations (60–100%) of inhaled oxygen to quickly achieve arterial PaO2 >80 mmHg and AaDO2 <250 mmHg, which is the mechanical ventilation strategy of early use (ventilator) and early removal (ventilator), within 72 h of onset. This strategy can rapidly improve hypoxia and increase the anti-inflammatory response.
Renal functions
Differential diagnoses of prerenal and intrarenal oliguria by fluid challenge should be completed within 30 minutes of admission. Additionally, it is necessary to actively treat the high IAP to avoid further damage to the kidneys (27). When the patient is diagnosed with acute renal failure, it is necessary to actively prevent major bleeding (surgical bleeding and wound oozing) in the abdominal cavity during the infection phase to improve the survival rate. The prevention strategy includes the following three aspects. First, vasoconstriction must be improved and kidney damage must be prevented with a continuous intravenous bolus injection of vitamin C at a dose of 200 mg/kg/h (28). Second, ulinastatin can significantly inhibit the elastase released from neutrophils; therefore, a daily dose of 1 million units/day should be administered using a continuous intravenous infusion for at least 2 weeks. One additional week of administration is required during the perioperative period. Third, before renal function can be recovered, the daily serum creatinine must be controlled to <300 µmol/L. These three measures are effective for preventing vascular bleeding and wound oozing after surgery for patients with acute renal failure. To date, there has been no report of how to prevent the acute response phase of SAP (whether renal function is restored) from being complicated by IAH after acute renal failure complications; however, our proposed strategy can significantly block wound oozing, with the preliminary results showing that it has significant efficacy (29). In addition, external biliary drainage plays a role in protecting the kidneys (30); therefore, it is of great significance to perform biliary drainage as early as possible during the acute response phase.
Intestinal function
Defecation is required within 24 h of admission. First, a warm-water enema (intermittent infusion of 2,000 mL within 24 h) should be administered. Then, an intramuscular injection of neostigmine (total of 0.5–5 mg/day) should be administered to increase intestinal motility; however, attention should be given to the heart rate, especially if it slows. When defecation does occur, atropine should be administered to enhance it; in addition, the Chinese medicine Dachengqi Decoction can be injected in the upper digestive tract, especially the nasojejunal tube, if possible (31). An intravenous injection of large doses of vitamin C (10 g/day) should be administered to protect the intestinal tract (32). Early use of an external biliary drainage can significantly protect the intestinal function (33).
Endocrine function
Stress hyperglycemia is inevitable, and even nonketotic hyperosmolar coma can occur. Increased blood glucose within a certain range is beneficial to the body, but an excessive increase causes damage to the body; therefore, it is necessary to strictly control blood glucose to 150 to 200 mg/dL within 6 to 12 h (34). For euthyroid sick syndrome (ESS) (35), also known as low T3 syndrome, thyroxine tablets are prohibited. However, when the T3 level is extremely low, as in the case of fulminant pancreatitis, appropriate replacement therapy using thyroxine tablets is recommended but controversial.
Nutritional support
Enteral nutrition is preferred. If the initial fluid resuscitation is appropriate to achieve the standard IAP of <20 mmHg, and if the intestinal tract has been cleared, then enteral nutrition must be initiated within 3 to 5 days of onset, but no later than within 7 days. The purpose of early initiation of enteral nutrition is to protect the intestinal mucosa from gut-derived sepsis; however, total enteral nutrition does not need to be rigorously applied. Insufficient calories can be supplemented via intravenous nutrition, but hyperlipidemic pancreatitis prohibits intravenous infusion of fat emulsions. When the conditions for enteral nutrition are fully satisfied, it should be administered gradually until total enteral nutrition is achieved. Nasal feeding is not recommended for patients with severe pancreatitis (36,37).
Antibiotics
There are two major debates regarding the use of antibiotics during the acute response phase: one argues whether the administration of antibiotics is for prevention or therapy, and the other argues whether it is necessary to administer prophylactic antibiotics (38). The use of antibiotics by patients with biliary pancreatitis complicated with acute cholangitis is therapy for biliary tract infection (39). Furthermore, not all guidelines recommend the prophylactic use of antibiotics for necrotic pancreatic tissue. However, animal experiments have shown that bacteria can translocate to the pancreatic tissue 15 minutes after the onset of SAP (40). On average, patients arrive to the emergency department 16 to 18 h after SAP onset; therefore, the so-called prophylactic antibiotic administration for pancreatic necrotic tissue is therapy, not prevention.
Based on our nearly 20 years of experience with antibiotic administration, we propose that early antibiotic administration should be conducted in a stepwise manner according to the etiology and severity of the disease.
Biliary pancreatitis
MAP can be treated with quinolones combined with metronidazole. Moderately severe acute pancreatitis (MSAP) should be treated with at least third-generation cephalosporin plus metronidazole or with fourth-generation cephalosporin. SAP should be treated with carbapenems, whereas fulminant pancreatitis should be treated with carbapenems, which are administered in combination with vancomycin or linezolid (41).
Non-biliary pancreatitis
MAP and MSAP are not treated with antibiotics; SAP is treated with third-generation cephalosporin plus metronidazole or with fourth-generation cephalosporins. Fulminant pancreatitis is treated with carbapenems. To date, there has been no indication implying cessation of antibiotic administration. In general, when the infection indices [body temperature, white blood cells, procalcitonin (PCT), C-reactive protein, lipopolysaccharides (LPS)] have returned to normal levels for 1 week and abdominal CT imaging has shown that the necrotic pancreatic tissue and intra-pancreatic and extra-pancreatic invasions have been completely capsulated, antibiotic administration may be ceased; this occurs generally 3 to 4 weeks after onset.
In summary, providing SAP patients with timely, reasonable, and effective treatment within 72 h of onset can significantly reduce complications, significantly shorten the course of disease, and reduce medical costs. The current research of the pathogenesis and aggravation mechanism of severe AP has not provided specific measures for completely preventing SAP from deteriorating (42). However, some patients experience iatrogenic SAP, which is caused by inappropriate medical intervention. Therefore, actively trying to solve the eight major problems during the acute response phase can achieve twice the results with half the effort.
Intensive management plan during the infection phase
During non-surgical treatment, catastrophic secondary complications are most likely to occur. Necrotic pancreatic tissue and/or uncontrolled infection due to extra-pancreatic invasion lead to sepsis or septic shock (43), resulting in prolonged hospitalization, increased treatment costs, and increased mortality. Similarly, in this phase, diagnosis and therapeutic interventions must be completed within the prescribed time according to the principles of emergency medicine.
Sources of infection and microbiology
The diagnostic process should be initiated immediately whenever a suspected infection occurs. Patients should immediately undergo plain and enhanced CT scans, with the scan ranging from the chest to the abdomen to the pelvis (at the pubic symphysis level).
Routine blood tests and PCT, LPS, and C-reactive protein examinations should be performed. In emergency situations, bacterial and fungal smears should be prepared from available body fluids (blood, urine, sputum, abdominal drainage fluid, bile), and the fluids should also be cultured for antimicrobial susceptibility testing. Within 24 h, it is imperative to have a determined a diagnosis of the source of infection and whether sepsis or septic shock is present.
Septic shock treatment
When septic shock occurs, it is imperative to immediately initiate controlled fluid resuscitation. The resuscitation strategies are the same as those used during the acute response phase with the exception that the target HCT is changed to 25% to 30% after fluid expansion.
Infection control
Surgical Infection
When the course of disease is less than 4 weeks, intensive management is preferred. For intra-abdominal and retroperitoneal infections that are beyond control after 48 h of intensive treatment, it is necessary to perform surgical intervention. Intensive management includes antibiotics. Carbapenems, vancomycin, or linezolid is administered with antifungal therapy. Hemoglobin and serum albumin levels are increased to 80 and 30 g/L, respectively, with infection. The criteria for uncontrolled infection include vital signs that continue to worsen and PCT, LPS, and white blood cell levels that continue to increase.
When the course of disease is more than 4 weeks, surgical interventions are performed within 24 h, including minimally invasive drainage (endoscope-guided or CT-guided catheterization) or direct drainage via laparotomy. Antibiotics are administered according to drainage smear results and microbiological evidence.
Infection related to surgery
When an infection is related to surgery, conservative management comprising drainage is performed. The central venous catheter is removed, and the sputum is discharged through a bronchoscope. Chinese medicine can be used to clear the intestine. Antibiotics are another option for surgery-related infections. According to the principles of microbiology, the broadest spectrum of pathogenic bacteria should be treated with the fewest antibiotics.
Visceral function maintenance
Central nervous system
For brain edema, mannitol should be administered to reduce intracranial pressure. For brain mitochondrial dysfunction (oxidative phosphorylation uncoupling), vitamin C 5 to 10 g/day should be administered. In the event of early detection of non-specific fungal encephalopathy, abnormal swallowing function, blurred vision, binocular gaze, or altered mental status, fluconazole should be administered with amphotericin B or pneumocandins.
Heart
Cardiac muscles can be depressed or stunned, and the B-type natriuretic peptide is significantly increased. Furthermore, the level of troponin is increased. There is sympathetic stimulation of the heart, but the interaction between endotoxins and myocardial hyperpolarized cyclic nucleotide ion channels leads to excessive excitation of cardiac pacemaker cells, leading to further sympathetic stimulation (44). Treatment includes improving the myocardial blood supply and energy metabolism. To improve energy, fibrin degradation products (FDP), sodium inositol phosphate, and the enzyme-coenzyme complex should be administered. Continuous instillation of nitroglycerin should be performed to improve myocardial blood supply, prevent arrhythmia, and increase the blood potassium level to 4 mmol/L. Statins, β-blockers, bisoprolol, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers should be administered to inhibit sympathetic stimulation to decrease the resting heart rate to <90 bpm (45).
Lungs
To prevent pulmonary infection, reduce hypoxia, and prevent recurrence of acute respiratory distress syndrome due to neutrophilic infiltration in the lungs, administer large doses of ulinastatin (e.g., continuous intravenous bolus injection at a dose of 1 million units/day) and perform open-lung ventilation (46).
Kidneys
Routine measurements of IAP should be performed to prevent recurrence of ACS. When septic shock occurs, controlled fluid resuscitation should be immediately initiated to prevent acute renal failure (47). After surgical drainage, it is imperative to start administration of methylprednisolone at a dose of 80 mg/day during the surgery. Methylprednisolone should be administered for 3 days to prevent infection-induced renal failure.
Intestinal tract
Enteral nutrition should be initiated again within 48 h after fluid resuscitation.
Liver and gallbladder
To protect the cell membrane, administer phosphatidylcholine. To eliminate cholestasis of bile capillaries, administer ademetionine and oxidized glutathione (GSSH). Administer sodium ursodeoxycholate to improve energy metabolism. Administer an enzyme-coenzyme complex when only the total bilirubin increases, and cease administration of somatostatin. Due to eating, hyperglycemia, and the administration of somatostatin, gallbladder gangrene is prone to occur during the infection phase (48).
Control persistent inflammation, immunosuppression, and catabolism syndrome (PICS)
Antioxidative stress management (49,50), intravenous bolus injection, administration of vitamin C (10–15 g/24 h), glutamine, and GSSH, as well as timely administration of albumin at a dose of 40 to 60 g/day should be performed. If serious PICS occurs postoperatively, then hemofiltration (high flow), short-term veno-venous hemofiltration (SVVH), and intermittent short veno-venuous hemofiltration (ISVVH) should be initiated immediately; the therapeutic dose is >45 mL/kg. High-dose ulinastatin (1 million U/24 h) should be added simultaneously. Agents enhancing cellular immunity (e.g., thymopentin) should be simultaneously administered as well (51).
Conclusions
The research protocol reported here is mainly based on our own basic and clinical research. During our more than 10 years of experience with a large number of clinical cases, significant clinical efficacy was shown. If the protocol is strictly implemented, then the treatment success rate could surpass 95%.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- De Waele JJ. Acute pancreatitis. Curr Opin Crit Care 2014;20:189-95. [Crossref] [PubMed]
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13:e1-15. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Wang JL, Chen Y, Song XQ, et al. Biliary tract external drainage protects against multiple organs injuries of severe acute pancreatitis rats via heme oxygenase-1 upregulation. Pancreatology 2017;17:219-27. [Crossref] [PubMed]
- Liu YJ, Mao EQ, Ouyang B, et al. Effect of biliary tract external drainage on cytokine expression and histomorphology of intestine, liver, and lung in rats with hemorrhagic shock. Crit Care Med 2009;37:2800-6. [Crossref] [PubMed]
- Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016;59:128-40. [Crossref] [PubMed]
- Yu W, Li W, Wang Z, et al. Early percutaneous transhepatic gallbladder drainage compared with endoscopic retrograde cholangiopancreatography and papillotomy treatment for severe gallstone associated acute pancreatitis. Postgrad Med J 2007;83:187-91. [Crossref] [PubMed]
- Scherer J, Singh VP, Pitchumoni CS, et al. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol 2014;48:195-203. [Crossref] [PubMed]
- Mao EQ, Tang YQ, Zhang SD. Formalized therapeutic guideline for hyperlipidemic severe acute pancreatitis. World J Gastroenterol 2003;9:2622-6. [Crossref] [PubMed]
- Clemens DL, Schneider KJ, Arkfeld CK, et al. Alcoholic pancreatitis: New insights into the pathogenesis and treatment. World J Gastrointest Pathophysiol 2016;7:48-58. [Crossref] [PubMed]
- Ducarme G, Maire F, Chatel P, et al. Acute pancreatitis during pregnancy: a review. J Perinatol 2014;34:87-94. [Crossref] [PubMed]
- Bai HX, Giefer M, Patel M, et al. The association of primary hyperparathyroidism with pancreatitis. J Clin Gastroenterol 2012;46:656-61. [PubMed]
- Mao EQ, Tang YQ, Fei J, et al. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl) 2009;122:169-73. [PubMed]
- Mao EQ, Fei J, Peng YB, et al. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl) 2010;123:1639-44. [PubMed]
- Yiqi D. Interpretation of the Guidelines for Diagnosis and Treatment of Acute Pancreatitis in China in 2013 ] Chin J Prac Intern Med 2014;34:859-61.
- Sun Y, Lu ZH, Zhang XS, et al. The effects of fluid resuscitation according to PiCCO on the early stage of severe acute pancreatitis. Pancreatology 2015;15:497-502. [Crossref] [PubMed]
- Sun W, Mao EQ. Comments on the article "Antioxidants as a treatment for acute pancreatitis: A meta-analysis". Pancreatology 2016;16:324-5. [Crossref] [PubMed]
- De Waele JJ, Cheatham ML, Malbrain ML, et al. Recommendations for research from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. Acta Clin Belg 2009;64:203-9. [Crossref] [PubMed]
- Sugrue M. Abdominal compartment syndrome. Curr Opin Crit Care 2005;11:333-8. [Crossref] [PubMed]
- Diuzheva TG, Shefer AV. Intra-abdominal hypertension in patients with severe acute pancreatitis. Khirurgiia (Mosk) 2014.21-9. [PubMed]
- Kirkpatrick AW, Roberts DJ, De Waele J, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013;39:1190-206. [Crossref] [PubMed]
- Hou J, Zhu MW, He XW, et al. Effect of hyperbaric oxygen and ulinastatin on plasma endotoxin, soluble CD14, endotoxin-neutralizing capacity and cytokines in acute necrotizing pancreatitis. Can J Surg 2010;53:241-5. [PubMed]
- Du WD, Yuan ZR, Sun J, et al. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J Gastroenterol 2003;9:2565-9. [Crossref] [PubMed]
- Chen H, Li F, Jia JG, et al. Effects of traditional Chinese medicine on intestinal mucosal permeability in early phase of severe acute pancreatitis. Chin Med J (Engl) 2010;123:1537-42. [PubMed]
- Mao EQ, Tang YQ, Zhang SD. Effects of time interval for hemofiltration on the prognosis of severe acute pancreatitis. World J Gastroenterol 2003;9:373-6. [Crossref] [PubMed]
- Tyberg A, Karia K, Gabr M, et al. Management of pancreatic fluid collections: A comprehensive review of the literature. World J Gastroenterol 2016;22:2256-70. [Crossref] [PubMed]
- Harman PK, Kron IL, McLachlan HD, et al. Elevated intra-abdominal pressure and renal function. Ann Surg 1982;196:594-7. [Crossref] [PubMed]
- Ma L, Fei J, Chen Y, et al. Vitamin C Attenuates Hemorrhagic Shock-induced Dendritic Cell-specific Intercellular Adhesion Molecule 3-grabbing Nonintegrin Expression in Tubular Epithelial Cells and Renal Injury in Rats. Chin Med J (Engl) 2016;129:1731-6. [Crossref] [PubMed]
- Huang J, Qu HP, Zheng YF, et al. The revised Atlanta criteria 2012 altered the classification, severity assessment and management of acute pancreatitis. Hepatobiliary Pancreat Dis Int 2016;15:310-5. [Crossref] [PubMed]
- Wang L, Zhao B, Chen Y, et al. Biliary tract external drainage alleviates kidney injury in shock. J Surg Res 2015;199:564-71. [Crossref] [PubMed]
- Chen Z, Chen Y, Pan L, et al. Dachengqi Decoction Attenuates Inflammatory Response via Inhibiting HMGB1 Mediated NF-kappaB and P38 MAPK Signaling Pathways in Severe Acute Pancreatitis. Cell Physiol Biochem 2015;37:1379-89. [Crossref] [PubMed]
- Ma L, Chen Y, Song X, et al. Vitamin C Attenuates Hemorrhagic Hypotension Induced Epithelial-Dendritic Cell Transformation in Rat Intestines by Maintaining GSK-3beta Activity and E-Cadherin Expression. Shock 2016;45:55-64. [Crossref] [PubMed]
- Wang L, Zhao B, Chen Y, et al. Biliary tract external drainage protects against intestinal barrier injury in hemorrhagic shock rats. World J Gastroenterol 2015;21:12800-13. [Crossref] [PubMed]
- Mentula P, Kylanpaa ML, Kemppainen E, et al. Obesity correlates with early hyperglycemia in patients with acute pancreatitis who developed organ failure. Pancreas 2008;36:e21-5. [Crossref] [PubMed]
- Stathatos N, Wartofsky L. The euthyroid sick syndrome: is there a physiologic rationale for thyroid hormone treatment? J Endocrinol Invest 2003;26:1174-9. [Crossref] [PubMed]
- Roberts KM, Nahikian-Nelms M, Ukleja A, et al. Nutritional Aspects of Acute Pancreatitis. Gastroenterol Clin North Am 2018;47:77-94. [Crossref] [PubMed]
- Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon 2012;10:350-6. [Crossref] [PubMed]
- Baltatzis M, Jegatheeswaran S, O'Reilly DA, et al. Antibiotic use in acute pancreatitis: Global overview of compliance with international guidelines. Pancreatology 2016;16:189-93. [Crossref] [PubMed]
- Oller Sales B, Rodriguez Conde N. Biliary tract infection: which antibiotics and in which setting. Med Clin (Barc) 2003;121:779-81. [Crossref] [PubMed]
- Zhang M, Zhu HM, He F, et al. Association between acute pancreatitis and small intestinal bacterial overgrowth assessed by hydrogen breath test. World J Gastroenterol 2017;23:8591-6. [Crossref] [PubMed]
- Norton ID, Clain JE. Optimising outcomes in acute pancreatitis. Drugs 2001;61:1581-91. [Crossref] [PubMed]
- Beger HG, Rau BM. Severe acute pancreatitis: Clinical course and management. World J Gastroenterol 2007;13:5043-51. [Crossref] [PubMed]
- Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol 2014;20:13879-92. [Crossref] [PubMed]
- Garg N, Soni KD, Aggarwal R. Unstable cardiac injury complicated with septic shock-a challenge. Burns Trauma 2016;4:11. [Crossref] [PubMed]
- Hori M, Okamoto H. Heart rate as a target of treatment of chronic heart failure. J Cardiol 2012;60:86-90. [Crossref] [PubMed]
- Wang L, Huang X, Kong G, et al. Ulinastatin attenuates pulmonary endothelial glycocalyx damage and inhibits endothelial heparanase activity in LPS-induced ARDS. Biochem Biophys Res Commun 2016;478:669-75. [Crossref] [PubMed]
- Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 2014;10:37-47. [Crossref] [PubMed]
- Chen EZ, Huang J, Xu ZW, et al. Clinical features and outcomes of patients with severe acute pancreatitis complicated with gangrenous cholecystitis. Hepatobiliary Pancreat Dis Int 2013;12:317-23. [Crossref] [PubMed]
- Zhao B, Fei J, Chen Y, et al. Pharmacological preconditioning with vitamin C attenuates intestinal injury via the induction of heme oxygenase-1 after hemorrhagic shock in rats. PLoS One 2014;9:e99134. [Crossref] [PubMed]
- Zhao B, Fei J, Chen Y, et al. Vitamin C treatment attenuates hemorrhagic shock related multi-organ injuries through the induction of heme oxygenase-1. BMC Complement Altern Med 2014;14:442. [Crossref] [PubMed]
- Liu D, Yu Z, Yin J, et al. Effect of ulinastatin combined with thymosin alpha1 on sepsis: A systematic review and meta-analysis of Chinese and Indian patients. J Crit Care 2017;39:285-7. [Crossref] [PubMed]


