Clinical characteristics and genetic testing of an atypical familial von Hippel-Lindauzon renal cell carcinoma
Introduction
Von Hippel-Lindauzon (VHL) syndrome (OMIM number 193300) is an autosomal dominant hereditary tumor susceptibility syndrome. Patients can be characterized as central nervous system hemangioblastoma, renal cell carcinoma (RCC), retinal hemangioma, renal tumor cyst, pancreatic tumor cyst, adrenal pheochromocytoma, inner ear lymphoma and epididymal cystadenoma (1), among which RCC is one of the most leading causes of death (2). The incidence rate varies from 1/35,000 to 1/40,000 among different ethnic groups, and the mean age of onset is 26 years old (3).
VHL gene, the disease-causing gene, is a tumor suppressor gene which is located at 3p25-26 (45 kb in length) and contains three exons (4). The encoded protein (pVHL) plays a crucial role in controlling the occurrence of vascular tumors, regulating the expression of cell growth-related genes and cell cycle. VHL gene mutations include point mutations, hypermethylation of the promoter, and loss of heterozygosity (LOH) due to single-copy deletion of large fragments of the gene (5). This article reviewed the diagnosis process of a patient with atypical VHL RCC in our hospital and discussed the clinical application value of VHL genetic testing.
Methods
Patients
Family
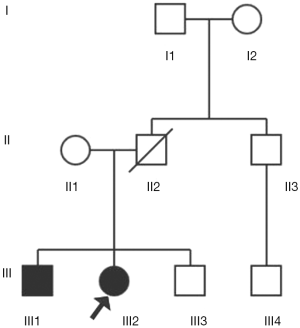
This study included 9 family members in three generations (details in Figure 1). The patient was the proband III2, and his brother III1 had a unilateral renal tumor history. A family member II2 with unidentified clinical characteristics had died of cirrhosis at age 42. All of the above surveys were based on a sound informed consent process (Details were seen at http://cdn.amegroups.cn/static/application/0a2c4d7ab700ed4f52541a5bc9e6b4a6.pdf).
Clinical data of the patient
The patient, female, was in hospital with a chief complaint of “physical examination revealed bilateral renal messes for one week”. The patient had symptoms of hypothermia, fatigue, no hematuria, and no percussion pain in the bilateral kidneys. She was severely obese [body mass index (BMI) =46.9 Kg/m2] with fatty liver. Her brother was also severely obese and had a history of unilateral renal tumor. CT examination revealed that she had bilateral renal messes and cysts appeared in the attachment areas on both sides. After definite diagnosis, she underwent partial nephrectomy of left kidney and the postoperative pathology was grade I clear cell carcinoma of the left kidney with tumor size of 4.5×3.5×3.5 cm. Three months later, radical resection of the right kidney was performed. The pathological findings showed that grade III clear cell carcinoma of the left kidney with extensive necrosis and the tumor volume was larger than that in left side. Nine months after the second surgery, multiple enlarged lymph nodes were found in mediastinum and hilum, metastasis was found in multiple organizations such as the bilateral adrenal glands, the bilateral lungs, the right femoral head, the iliac bone, the L3 vertebral body and so on.
Clinical imaging data
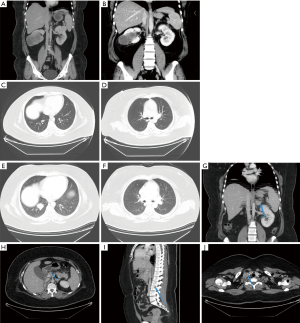
On December 21, 2017, CT scan revealed bilateral renal mass (about 3.6×3.5 cm for the left kidney mass and 6.3×7.3 cm for the right kidney mass) (Figure 2A). The results of CT examination of March 28, 2018 showed that the local tissue of the left kidney was densely depressed and changed for postoperative, which was a change after operation. The right renal mass shrank slightly, about 6.0×6.82 cm (Figure 2B). On July 2, 2018 (Figure 2C,D), CT findings showed that multiple pulmonary metastases, normal left kidney and absence of right kidney were all post-operative changes. On January 9, 2019 (Figure 2E,F,G,H,I,J), CT findings showed that multiple metastases in both lungs were larger than before; new nodules in the adrenal gland were seen and suspected to be metastasis; multiple lymph nodes in the right supraclavicular and mediastinum were enlarged; lymph nodes near the head of the pancreas were enlarged and suspected to be metastasis; bone destruction and metastasis occurred in the right femoral head, right iliac bone and the right side of L3 vertebral body.
Gene detection methods
Experimental materials
Peripheral blood samples were collected from proband, family members and normal controls. Genomic DNAs were extracted by phenol-chloroform method and RNAs were extracted using Trizol method.
PCR-DNA sequencing to detect base mutations, tiny insertion and deletion of base
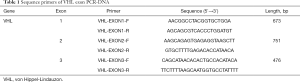
Primers were designed according to the exons of the VHL gene, and all coding sequences were amplified by PCR. The primer sequences are shown in Table 1. PCR reaction system (25 µL in total) contained 50–100 ng of genomic DNA, 0.2 mmol/L of dNTP, upstream and downstream primers were 0.5 µmol/L each and 2U of Taq HS DNA polymerase. PCR conditions: pre-denaturation at 94 °C for 5 min, 94 °C for 20 s, 58–62 °C for 40 s, 72 °C for 20 s, 35 cycles, 72 °C extension for 7 min. In addition, PCR products were detected by 2% agarose gel electrophoresis, and DNA fragments of PCR products were purified and recovered. Sequencing and analysis process were completed by specific company.
Full table
Fluorescence real-time quantitative PCR detection of VHL gene exon copy number variation
Real-time quantitative PCR was performed using the TaKaRa SYBR Premix EX TaqTM kit. The primer sequences are shown in Table 2. The 10 µL PCR reaction system contained 200 nM primers, 20 ng genome, and 5 µL SYBR Premix EX TaqTM. PCR reaction conditions: 95 °C for 1 min, 94 °C 5 s/60 °C for 20 s, 45 cycles, 3 parallel tubes were made for each sample and each experiment was repeated at least 3 times.

Full table
Data analysis was performed using the ΔΔCt method. Ct is the number of cycles when the fluorescence detection limit threshold is reached. Calculation formula: ΔΔCt = (Ct target gene-Ct internal reference gene) experimental group-(Ct target gene-Ct internal reference gene) control group. Relative copy number (experimental/control group) =2−ΔΔCt. The value of normal person is set to 1, and if 2−ΔΔCt >1.25, the copy number of the gene is considered to be increased, and when the value is less than 0.75, the number of copies of the base is thought to be decreased.
RT-PCR amplification sequencing to verify exon single copy deletion
The Vazyme HiScript®IIReverse Transcriptase kit was used to reverse-transcribe the RNA samples, and the cDNAs obtained from reverse transcription were used as templates for PCR. The upstream and downstream primers were designed in the exon1 and exon3 regions respectively. Relevant sequences are shown in Table 3. Amplification length was amplified using TaKaRa LA Taq® with GC Buffer. The 50 µL PCR reaction system included 500 nM primers, 0.5 µL TaKaRa La Taq, 40 ng cDNA, 8 µL dNTP Mixture and 25 µL Buffer II. PCR conditions: pre-denaturation at 94 °C for 5 min, 94 °C for 20 s, 56 °C for 30 s, 72 °C for 20 s, 35 cycles, 72 °C extension for 7 min. PCR products were detected by 1.5% agarose gel electrophoresis, and DNA fragments of PCR products were purified and recovered. Furthermore, PCR products were sent to the company for further sequencing and analysis.

Full table
Results
PCR-DNA sequencing analysis results
PCR-DNA sequencing was used to detect point mutations in the VHL gene and analysis results showed that no point mutations were detected in the coding region of the VHL gene in the whole family member.
Results of Fluorescence real-time quantitative PCR detection
In normal people, the number of cycles needed to reach the threshold for exon 2 amplification of VHL gene is less than that required for the internal reference gene Hsa21, indicating that the number of copies of exon 2 in normal human gene is more than that of Hsa21. However, in patient samples, the number of cycles needed to reach the threshold for exon 2 amplification of VHL gene is less than that required for Hsa21 indicating that the number of copies of exon 2 in patient gene was less than that of Hsa21 (Figure 3A). Therefore, the copy number of exon2 gene in the peripheral blood of patients was less than that of normal people.
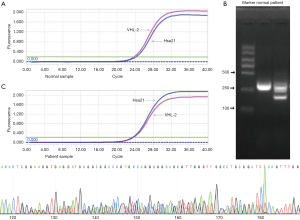
Data analysis was performed using ΔΔCt method and results showed that the copy number of the VHL gene exon 2 in this patient was 0.62 when compared to normal control, which indicated decrease in copy number of the VHL gene exon 2.
Results of agarose gel electrophoresis
PCR products of cDNA as templates were obtained by reverse transcription. 2% agarose gel electrophoresis is shown in Figure 3B. The length of the normal human product fragment should be 332 bp. However, there were two bands of cDNA products in agarose gel electrophoresis of the patients, about 332 and 209bp in length, respectively, which was in line with the VHL gene exon2 heterozygous deletion.
Sequencing results of DNA template after PCR
The sequencing of PCR products revealed that the peak appeared in exon2, and the length of the deletion fragment was 123 bp (Figure 3C).
Diagnostic criteria for VHL syndrome
Melmon and Rosen (6) proposed the diagnostic criteria for VHL syndrome in 1964 for the first time. They regarded cerebellar hemangioblastoma as a marker of tumors associated with VHL syndrome. VHL syndrome can be diagnosed when the patient had cerebrovascular blastoma and any of the following diseases: retinal hemangioblastoma, pancreatic cyst, abnormal kidney or epididymis. Afterwards, with the recognition of the genetic characteristics of the disease, individuals with central nervous system hemangioblastoma and one of the aforementioned diseases among family members were also included in the clinical diagnostic criteria for VHL syndrome.
Current diagnostic criteria for VHL syndrome were usually composed of family genetic history and clinical manifestations. Patients with a family history of retinal hemangioma or central nervous system hemangioblastoma can be diagnosed as long as they have either retinal hemangioma, central nervous system hemangioblastoma or substantial organ damage (RCC, multiple pancreatic cysts or tumors, pheochromocytoma, papillary cystadenoma of the epididymis). For those who had no family history, more than two (including two) hemangiomas or one hemangioma and one solid organ tumor were needed to make diagnosis.
Discussion
VHL syndrome, also known as Hippel-Lindau syndrome, is an autosomal dominant hereditary tumor syndrome involving multiple systems (7). VHL RCC is the most common hereditary RCC, often occurring in young people and the current average age of death in China is 62 years (8).
pVHL, encoded by wild genes in normal cells, is a ubiquitin ligase of hypoxia inducible factor HIF-1, which can induce the ubiquitination degradation of HIF-1 and inhibit the transcription of a series of genes involved in hypoxia and other tumorigenesis processes mediated by HIF-1 (9). Evidence had shown that wild-type pVHL also had multiple functions, including participation in the formation or assembly of extracellular fibronectin matrix, regulation of cell cycle and so on. Loss of these functions can promote tumorigenesis.
With the discovery of VHL suppressor gene in 1993, genetic testing had become the gold standard for the diagnosis of VHL syndrome. The diagnosis of VHL syndrome by mutation genetic testing is superior to that based on clinical manifestations. Genetic testing is particularly important for patients with concealed symptoms, incomplete genetic spreads, and no clear family history. Therefore, atypical VHL RCC can be diagnosed according to patient's clinical manifestations and peripheral blood gene mutation test results.
Peripheral blood of the patients was collected and DNA was extracted. PCR-DNA sequencing, real-time quantitative PCR and other methods were used to detect base mutations, tiny insertion and deletion of base, and large fragment deletion of VHL gene. Results showed that the single copy deletion of exon 2 of VHL gene in this patient was a pathological embryonic mutation, and no embryonic mutation was detected in other family members. Studies have shown that the protein encoded by this large fragment deletion gene is pVHL172 (10). It had already been proved that pVHL172 was not a tumor suppressor and the stable expression of pVHL172 in RCC cells could not regulate the level of HIV-1, which resulted in increased expression of angiogenic factors, decreased apoptosis rate and increased cell proliferation rate, and tumorigenesis. pVHL172 is not only a dominant inactivation of wild-type proteins, it also can induce higher sarcomatoid metastasis than parental cells that do not express pVHL.
The pathogenic gene follows the Knudson’s two-hit hypothesis (11). When VHL alleles were mutated and inactivated, tumors began to form. Therefore, VHL syndrome was divided into familial type and sporadic type. The examination results showed that the elder brother of the patient only had unilateral RCC and no germline mutation was detected in VHL, which was belonged to sporadic type. However, the patient was bilateral ccRCC, which developed rapidly, and metastasis was found in many tissues during follow-up. In contrast, familial VHL RCC has a higher incidence, higher possibility of bilateral tumors, early onset, prone to new lesions and multiple metastases, and poor prognosis when compared to sporadic RCC (11).
Virtue et al. found that HIF-1 induced inflammation and insulin resistance in obesity. In addition, abnormal regulation of HIF complexes played a promoting role in the development of obesity, fatty liver and type 2 diabetes (12). Both the patient and his brother were severely obese, and the patient was also diagnosed with fatty liver, which was associated with a decrease in the breakdown of HIF-1 due to mutations in its VHL gene. Nowadays, gene-targeted drugs therapy has been widely used in the treatment of advanced RCC and many dysregulated signaling pathways in RCC had been demonstrated, drugs for these pathways had initially shown promising results in clinical use. For example, axitinib, cabotinib, pazopanib, sorafenib and sunitinib developed according to the pathway in which the downstream components of the VHL-HIF-VEGF pathway are regulated by receptor tyrosine kinase inhibitors; mTOR inhibitors (ivermox, sirolimus, etc.) which were aimed to reduce the accumulation of HIF protein (13).
Conclusions
Detection of VHL gene mutation in atypical VHL RCC family can played an important role in detecting asymptomatic patients and pathogenic gene carriers at an early stage, providing solutions for clinical decision-making and management, which is of great significance for prolonging the survival time of patients and improving the quality of life.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No.81702520), Medical Research Project of Jiangsu Provincial Health and Family Planning Commission (No. H2018052), Research Project of Jiangsu Cancer Hospital (No. ZN201602), and the young talents program of Jiangsu Cancer Hospital (No. 2017YQL-04).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board (IRB) in Jiangsu Cancer Hospital (No. 2019-011), and informed consent was obtained from all patients before present study initiated.
References
- Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 1993;260:1317-20. [Crossref] [PubMed]
- Chauveau D, Duvic C, Chrétien Y, et al. Renal involvement in von Hippel-Lindau disease. Kidney Int 1996;50:944-51. [Crossref] [PubMed]
- Peng S, Shepard MJ, Wang J, et al. Genotype-phenotype correlations in Chinese von Hippel-Lindau disease patients. Oncotarget 2017;8:38456-65. [PubMed]
- Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol 2004;22:4991-5004. [Crossref] [PubMed]
- Kim JA, Choi DK, Min JS, et al. VBP1 represses cancer metastasis by enhancing HIF-1alpha degradation induced by pVHL. FEBS J 2018;285:115-26. [Crossref] [PubMed]
- Melmon KL, Rosen SW. Lindau's Disease. Review of the Literature and Study of a Large Kindred. Am J Med 1964;36:595-617. [Crossref] [PubMed]
- Kaelin WG Jr. The von Hippel-Lindau tumor suppressor gene and kidney cancer. Clin Cancer Res 2004;10:6290S-5S. [Crossref] [PubMed]
- Wang JY, Peng SH, Li T, et al. Risk factors for survival in patients with von Hippel-Lindau disease. J Med Genet 2018;55:322-8. [Crossref] [PubMed]
- Chintala S, Najrana T, Toth K, et al. Prolyl hydroxylase 2 dependent and Von-Hippel-Lindau independent degradation of Hypoxia-inducible factor 1 and 2 alpha by selenium in clear cell renal cell carcinoma leads to tumor growth inhibition. BMC Cancer 2012;12:293. [Crossref] [PubMed]
- Hascoet P, Chesnel F, Jouan F, et al. The pVHL172 isoform is not a tumor suppressor and up-regulates a subset of pro-tumorigenic genes including TGFB1 and MMP13. Oncotarget 2017;8:75989-6002. [Crossref] [PubMed]
- Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet 2003;361:2059-67. [Crossref] [PubMed]
- Virtue S, Vidal-Puig A. Nothing Iffy about HIF in the Hypothalamus. PLoS Biol 2011;9:e1001116. [Crossref] [PubMed]
- Kim E, Zschiedrich S. Renal Cell Carcinoma in von Hippel-Lindau Disease-From Tumor Genetics to Novel Therapeutic Strategies. Front Pediatr 2018;6:16. [Crossref] [PubMed]