Vortioxetine versus sertraline in metabolic control, distress and depression in Mexican patients with type 2 diabetes
Introduction
Type 2 diabetes (T2D) and depression are chronic diseases with increasing prevalence worldwide due to lifestyle modifications and increasing lifetime expectancy. Moreover, both illnesses can occur simultaneously (1). Individuals with T2D have twice the risk of suffering depression (2). This combination of T2D and depression reflects an interaction of common multifactor etiology, which not only includes lifestyle changes and diabetes-associated stress (3,4), but also dysregulation of biological systems related to pro-inflammatory state, insulin resistance, and stress hormones (5,6).
Diagnosis of T2D and depression are commonly unnoticed and untreated (7,8). This situation leads to a strong negative impact on the quality of life of those being affected. In addition, depression in patients with T2D is associated with poor glycemic control and negative health-related outcomes in diabetes (weight gain, lack of adherence to therapy and long-term diabetic macrovascular and microvascular complications) (9-11).
Antidepressants are first line drugs for the treatment of mood disorders (depression, among them). However, several reports have demonstrated that depression treatment in patients with T2D may be ineffective given that selective serotonin reuptake inhibitors and selective serotonin and norepinephrine reuptake inhibitors are associated with metabolic dysregulation. These alterations produce increased in fasting blood glucose, glycated hemoglobin (HbA1c), insulin resistance, and LDL-cholesterol and triacylglycerol levels, among the most common impairment outcomes. Furthermore, there is evidence that suggests high body mass index (BMI) and abdominal obesity (12,13). Sertraline, a selective inhibitor of the serotonin receptor (SSRI), has been described to remit depression (14), together with other additional effects such as increases in insulin secretion, cholesterol, and triacylglycerol levels, as well as inducer of loss of appetite in depressed patients with T2D (15,16). These biochemical and clinical parameters can lead to the prescription of new pharmacological alternatives for the treatment of depression in patients with T2D, that may promote both the remission of depression and glycemic control. In this sense, recent studies indicate that the antidepressant vortioxetine is a modulator and simulator of serotonin since it exerts a multimodal mechanism of action toward the serotonergic neurotransmitter system. This antidepressant has shown less adverse effects and increased cognitive function (17).
The aim of this study was to determine the efficacy of vortioxetine versus sertraline in the remission of depression, diabetes-related distress, anthropometric measures and the influence of these drugs in glycemic control in patients with T2D and depression in a Mexican population.
Methods
Participants
This was a randomized single-blind study in which the same medical practitioner in the clinic of diabetes assigned at random to the patients to each of the two groups (vortioxetine and sertraline treatments). They were simply randomized into 2 groups by a computer-generated list of random numbers; staff of Regional Hospital of High Specialty Dr. Gustavo A. Rovirosa Pérez, who did not know in detail the research, enrolled participants and assigned participants to interventions. Twenty-one participants (Vortioxetine: 12; Sertraline: 9) completed the study, there were 28 dropouts (Vortioxetine: 11; Sertraline: 17). The treatment evaluation was conducted by the psychiatrists, investigators, and patients participating in the study who were blinded to the diabetes treatment. The study was conducted from June 2017 to June 2018. Participants were recruited from the Clinic for Diabetes in the third-level of the Regional Hospital of High Specialty Dr. Gustavo A. Rovirosa Pérez in Villahermosa, Tabasco, southeastern Mexico.
Inclusion criteria were the following: (I) individuals had to be between 18 and 60 years of age, (II) diagnosis of T2D based on the American Diabetes Association criteria, (III) individuals with glycosylated hemoglobin ≥7.5%, (IV) subjects had to be under anti-diabetic treatment, (IV) diagnosis of major depressive episode in accordance with the Diagnostic and Statistical Manual of Mental Disorders fifth edition (DSM-5) and rating scale score ≥14 in the Hamilton Depression scale (HAM-D), and (V) patients had to give with verbal and written informed consent to participate in this study. Exclusion criteria were: patients diagnosed with a concomitant neurological illness or under psychoactive medications, patients showing clinical parameters of type I diabetes, or patients exhibiting active suicidal ideation.
Ethical statement
Participation was voluntary; patients were informed about the objectives and aims of the research. Patients signed a written informed consent before the interview. The study was approved by the hospital ethics committee (00228/16). This investigation was carried out at the Clinic for Diabetes in collaboration with the Department of Psychiatry.
Clinical measures
The assessment consisted of an extended face-to-face interview. Patients answered a structured questionnaire to collect socio-demographic characteristics (gender, age, education, occupation, and marital status) and clinical data (pharmacotherapy, habits, drug consumption, diabetes complications, and comorbidities). Depression was screened using the HAM-D instrument, Center for Epidemiologic Studies Depression Scale revised in Spanish (CES-DR35), and Problem Areas in Diabetes Questionnaire (PAID-V). Besides, we evaluated the remission of depression post-treatment with HAM-D scale rating score ≤7 (18,19). We also assembled anthropometric measurements: weight, waist circumference, and BMI. Besides, blood samples were drawn to assess the following biochemical parameters: fasting plasma glucose (FPG), HbA1c, and cholesterol and triacylglycerol levels.
Subsequently, patients who provided an informed consent and convened with the inclusion criteria were attended by a psychiatrist. Pharmacological management was randomly assigned blind to the participant: vortioxetine (10 mg/day) or sertraline. The latter was initially 50 mg/day and in all the subjects the dose was adjusted starting from day 7 into 100 mg/day until the end of the study. The antidepressant treatment was administered for 8 weeks (20). In addition, patients maintained their established anti-diabetic treatment (oral hypoglycemic or insulin). Subsequently, patients were interviewed by a psychiatrist, followed by the extraction of blood samples to assess the biochemical parameters mentioned above and the filling out of a structured questionnaire (previously described). Clinical measures and biochemical parameters were taken at two different stages: prior to antidepressant treatment and after the 8-week pharmacological treatment (12,13).
Depression diagnosis and evaluation and diabetes-specific stress
The assessment of depression was performed by the HAM-D rating scale validated in the Spanish version (21). We used a 17-item reduced version, the range of values for the scale was 0–50, where 14 was the cutoff point; patients with this score were diagnosed with major depression. The severity of depression was measured with the CES-DR35 scale (22). Previous studies in a Mexican population reported a Cronbach α score of 0.9 (22). The CES-DR35 questionnaire consists of 35 items, which includes depressed mood, anhedonia, appetite, sleep problem, psychomotor retardation, fatigue, guilt/conscience, thinking, suicidal ideation and social. These items measure symptoms defined by DSM-5, and were the ones assessed in the present study. In addition, diabetes-specific distress was assessed with the PAID-V instrument. This scale consists of 5 items. The total score ranges from 0 to 20. Positive diagnosis was considered for scores ≥8 to indicate greater emotional stress (23).
Body weight, BMI and anthropometric parameters
We measured weight, height and BMI. The latter was calculated as weight in kilograms divided by the square of the height in square meters (24) Waist circumference was defined as the smallest circumference between the lower rib and the iliac crest (25).
Biochemical parameters
We assessed the following biochemical parameters: FPG, and cholesterol and triacylglycerol levels. These parameters were analyzed with the Clinical Chemistry System from Random Access Diagnostics. Glycated hemoglobin was determined by an enzymatic immunoassay method.
Statistical analysis
Characteristics of sample are expressed as mean ± standard deviation (SD). Patients were included in a multivariate analysis of variance (MANOVA) model to examine changes in time (time effect), between treatment groups (interaction effect), and treatment x time as the effects of interest. The primary efficacy parameter was the severity of depressive symptom (CES-DR35 items and HAM-D total score), whereas secondary parameters included diabetes specific-stress, as well as anthropometric and biochemical variables. The partial eta squared (ƞ2p) was used as an estimator of the effect size of comparisons, with the following values for interpretation: 0.02= small, 0.02–0.09= medium, >0.09= large (26). Statistical analyses were performed using the Statistical Package for Social Sciences software (SPSS, Chicago, IL, USA). The significance cutoff value was set at P≤0.05.
Sample calculation was obtained through the G*Power 3.1 program. Considering an a priori power analysis for differences between two marched pairs and assuming equal variances with bilateral hypotheses, with a statistical power of 0.95, and effect size (d) of 0.85, a total of 21 patients were needed for the present study.
Results
Socio-demographic parameters of subjects with T2D and depression
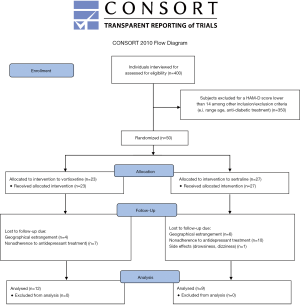
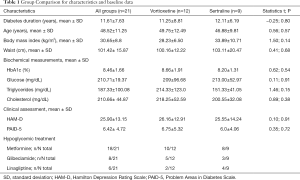
A total of 400 patients with T2D were interviewed. Fifty subjects met the depressed-inclusion criteria for the study. Given the high withdrawal rate, only 21 finished the treatment (Figure S1). The majority of participants with T2D and depression were female (71.4%, n=15); age 48.52±11.25 years old, level of education 8.52±4.2 years, 71.4% (n=15) were married. The main occupations were classified as part-time jobs (42.9%, n=9) and household chores (57.1%, n=12). Patients with T2D came predominantly from a low economic status. Average duration of diabetes was 11.61±7.63 years and BMI was 30.65±8.8 kg/m2. From the 21 patients included in the study, 57.1% (n=12) were treated with vortioxetine and the remaining 42.9% (n=9) with sertraline. The comparison of the baseline characteristics among groups are shown in Table 1. As can be seen, both groups displayed similar demographic and baseline clinical features. Subjects with T2D and depression who dropped out of the study (n=29) did so for the following reasons: non-adherence to antidepressant treatment (n=18), failure to attend the second interview due to home remoteness from hospital (n=10), and presence of side effects such as drowsiness and dizziness (n=1).
Full table
Depression in subjects with T2D
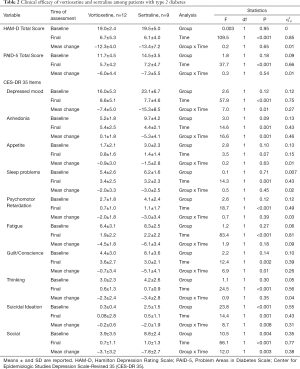
The remission of depression in T2D patients and depression after 8 weeks of treatment was 90.47% (n=19) for vortioxetine (n=11) and sertraline (n=8); remission rates were similar in both groups (P=0.72). Both antidepressants were effective in reducing depression (HAM-D) and diabetes-related stress and almost all symptoms assessed with the CES-DR35 except appetite, in which no significant improvements in time were observed in both groups. In the case of anhedonia only, no differences in time were observed in the group treated with vortioxetine. Suicidal ideation and social were more severe at baseline values in the group treated with sertraline; however, these two symptoms along with depressed mood, anhedonia and guilt/conscience exhibited a better improvement in time in patients under sertraline treatment when compared to those of vortioxetine. These results are presented in Table 2, as well as the effect sizes of each comparison.
Full table
Biochemical parameters and anthropometric characteristics in subjects with T2D and depression
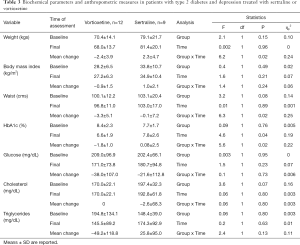
Both groups displayed similar biochemical parameters and anthropometric characteristics at baseline values without differences in time and by groups during the treatment period in BMI, glucose, and cholesterol levels, but not for triglycerides. Nevertheless, it was observed that patients treated with vortioxetine showed significant reductions in body weight, waist circumference, and HbA1c, whereas in those treated with sertraline, waist and HbA1c remained the same and body weight increased. The comparison of these parameters is shown in Table 3.
Full table
Discussion
The aim of this study was to determine the effect of vortioxetine versus sertraline on the remission of depression, as well as the influence of these antidepressants on glycemic control in patients with T2D and depression in a Mexican population.
Remission of depression in subjects with T2D and depression
We recognize that sertraline and vortioxetine were effective to remit depression (Ham-D and CES-DR35) and diabetes-related stress in patients with T2D. Our findings are consistent with other reports in which vortioxetine showed similar efficacy in the remission of depression as that for sertraline, agomelatine, desvenlafaxine, duloxetine, escitalopram, venlafaxine and vilazodone (27). Previous studies in patients with T2D and depression treated with sertraline have reported improvement in depressive symptoms and emotional stress related to diabetes demonstrated, in subjects with T2D and depression treated with sertraline for 12 weeks a decrease in depression symptoms (28,29). On the other hand, vortioxetine improved depressive symptoms with respect to placebo after 6–8 weeks of treatment (30). To our knowledge, our current study is the first to compare sertraline versus vortioxetine. We found that sertraline revealed a better effect compared to vortioxetine in depression when we analyzed all items of CES-DR35. Previous studies suggest that the CES-DR35 scale is rather comprehensive since it considers the following parameters: negative mood, emotional stress, anxiety symptoms, subclinical depression, substance abuse, and general disorders. The majority of patients with diabetes and high levels of depressive symptoms are not clinically depressed. Besides, diabetes encompasses more general emotional distress and specific diabetes symptoms than clinical depression (31,32).
It is known that depression in patients with diabetes is associated with emotional stress, lack of glycemic control and medication adherence, complications related to diabetes and poor quality of life (33). Several reports have revealed that vortioxetine is associated with amelioration in cognition, psychomotor speed, executive function, improvements in sleep, energy and appetite in patients with depression without T2D (34) Similarly, this drug reduces acute depressive episodes and relapse rates of depression in subjects without diabetes (35).
Pharmacological and biochemical studies suggest that vortioxetine anti-depressive activity acts by increasing serotonergic neurotransmission, participation of 5-HT7 receptor antagonist, partial agonist activity towards 5-HT1B receptors and inhibition of serotonin reuptake (35,36). Mork et al., demonstrated that vortioxetine increases, in a dose-dependent manner, extracellular concentrations of 5-HT, dopamine, acetylcholine, and histamine in prefrontal cortex, whereas agonists of the 5-HT1B receptor increase the firing rate of serotonergic neurons in the raphe nucleus in rats (37). Other reports showed that vortioxetine, antagonist of the 5-HT3A receptor, increases serotonin, dopamine, noradrenaline, acetylcholine, and histamine concentrations in areas associated with depression (hippocampus and prefrontal cortex) (38).
Anthropometric characteristics of subjects with T2D and depression
Our work establishes that vortioxetine causes no weight gain in subjects with T2D and depression with respect to sertraline. This outcome promotes glycemic control in patients. In general, antidepressants are associated with weight gain, which in turn may predispose individuals to develop T2D. Subjects who have used tricyclic antidepressants and selective serotonin reuptake inhibitors for 2 years or longer double their risk for developing diabetes than those who do not take antidepressants (2,39). Some reports have revealed no significant effect of vortioxetine on body weight at 6-, 8- or 12-week placebo-controlled studies. In contrast, one study reported weight loss (40). It is known that vortioxetine acts by serotonin reuptake blockage and direct modulation (agonist and antagonist) of several serotonin receptors (35). Vortioxetine action on 5-HT1B and 5-HT2C receptors may reduce appetite and weight by generating early pre-meal satiety and less caloric intake (41).
It is known that antidepressants may induce weight gain by interfering with central nervous functions and energy balance regulation. In contrast, bupropion weight loss has been identified with blockage of dopamine and norepinephrine reuptake, but no effect on adrenergic, histamine or serotonergic receptors (16). However, altered metabolic rate may explain weight gain in depressed patients despite reduced appetite (42). According to this study, appetite and feeding are regulated by the complex participation of neurotransmitters, neuromodulators, cytokines and hormones interacting within the hypothalamus (leptin and tumor necrosis factor system). Serotonin participates in appetite and the regulation of satiety. Therefore, serotonin and histamine antagonists lead to central feeding increase and may account for weight gain in subjects treated with antidepressants (tricyclic antidepressants, selective serotonin reuptake inhibitor, and monoaminoxidase-inhibitors). However, different drugs exert distinct activities given the diversity of receptor systems and genetic predisposition (2,43).
Biochemical parameters in subjects with T2D and depression
In our study, vortioxetine ameliorates biochemical parameters (HbA1c, FGB, cholesterol and triacylglycerol) in T2D patients with depression. Depressed patients showed sensitivity to stress; hence glucose homeostasis could be more affected than in non-diabetic individuals. In agreement with our data, several authors have determined that SSRIs may be effective in reducing depressive symptoms and improving better glycemic control in people with diabetes and depression (44). Several studies have reported that chronic fluoxetine treatment diminishes blood glucose and lipids in patients without T2D. However, imipramine treatment increases serum total cholesterol, triacylglycerols, insulin resistance and body weight gain in depressive patients (12,42).
In the literature non-diabetic patients with depression treated with sertraline for 12 weeks presented no significant differences in glucose and HbA1C concentrations, but triacylglycerol levels increased on week 8 (15). Patients with T2D and depression treated with Cognitive Behavioral Therapy improved HbA1c when compared to sertraline treated patients (28). Serotonin receptor blockade at nerve terminals may participate in glucose homeostasis and inhibition of adrenaline release through 5-HT receptors in humans and animals. This adrenal gland release regulates and elevates blood glucose levels by inhibiting insulin release, glycolysis, or glucose uptake in tissues (12). Besides, antidepressants may inhibit synaptic reuptake of norepinephrine activating glycogenolysis and gluconeogenesis, which will cause raised blood glucose levels and insulin resistance. Different studies have reported that sertraline and fluoxetine reduce serum glucose levels in diabetic and non-diabetic animals; this may be related to higher insulin release, insulin receptor sensitivity, and decreased gluconeogenesis, but no effect on HbA1c. In contrast, nortriptyline increases blood glucose levels in non-diabetic and diabetic mice (45).
Furthermore, dyslipemia is associated with antidepressant use (46). This study showed that vortioxetine reduces cholesterol and triacylglycerols in subjects with T2D and depression, whereas sertraline treatment raises triacylglycerol concentrations. In this sense, some authors have reported that antidepressants may activate sterol regulatory element-binding protein transcription factors and up-regulate genes for cholesterol and fatty acid biosynthesis (47).
Limitations
In this research we acknowledge the following limitations: first, patients received the treatment (antidepressants) for 8 weeks only. Second, we observed a lack of adherence to antidepressant medication; as a result, those patients were withdrawn from the study. Third, even though we collected information regarding the treatment-adherence of antidepressants and hypoglycemic agents; these data were not evaluated in the effect of sertraline and vortioxetine. Furthermore, we discarded individuals with side effects as drowsiness and dizziness (Figure S1); therefore, the outcome of side effects on the groups of vortioxetine and sertraline was not analyzed. Additionally, the identification of depression remissions factors in this sample would be an important result to add to the manuscript. Unfortunately, the sample size was the main limitation to perform a multivariate regression analysis as no variability was observed in terms of HAM-D total score change (−12.3 vs. −13.4), as well as the number of patients in each group who were still depressed at the end of the study (one patient per group). Finally, the size of the sample is small, hence this study should be considered a pilot study.
Conclusions
We observed that vortioxetine and sertraline presented the same effect in the remission of depression and diabetes-related stress in patients with T2D and depression. However, vortioxetine improved glycemic control and showed a tendency to decrease anthropometric parameters (abdominal circumference and BMI) in patients with T2D and depression in a Mexican population. We suggest that vortioxetine improves metabolic control in T2D patients and does not interfere with the metabolism of carbohydrates. We suggest that pharmacological treatment with antidepressants combined with hypoglycemic drugs may prevent diabetic complications in patients with T2D and depression.
Acknowledgments
We greatly appreciate the collaboration of MD Rebeca Hernandez Martinez in clinical analysis laboratory (DACS-UJAT).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the hospital ethics committee (00228/16).
References
- Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med 2002;347:1342-9. [Crossref] [PubMed]
- Andersohn F, Schade R, Suissa S, et al. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry 2009;166:591-8. [Crossref] [PubMed]
- Sartorius N. Depression and diabetes. Dialogues Clin Neurosci 2018;20:47-52. [PubMed]
- Dziemidok P, Makara-Studzinska M, Jarosz MJ. Diabetes and depression: a combination of civilization and life-style diseases is more than simple problem adding - literature review. Ann Agric Environ Med 2011;18:318-22. [PubMed]
- Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci 2017;1391:20-34. [Crossref] [PubMed]
- Kohlgruber A, Lynch L. Adipose tissue inflammation in the pathogenesis of type 2 diabetes. Curr Diab Rep 2015;15:92. [Crossref] [PubMed]
- Hermanns N, Caputo S, Dzida G, et al. Screening, evaluation and management of depression in people with diabetes in primary care. Prim Care Diabetes 2013;7:1-10. [Crossref] [PubMed]
- Katon WJ. The comorbidity of diabetes mellitus and depression. Am J Med 2008;121:S8-15. [Crossref] [PubMed]
- Atif M, Saleem Q, Babar ZU, et al. Association between the Vicious Cycle of Diabetes-Associated Complications and Glycemic Control among the Elderly: A Systematic Review. Medicina (Kaunas) 2018;54. [Crossref] [PubMed]
- Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care 2003;26:2822-8. [Crossref] [PubMed]
- Ciechanowski PS, Katon WJ, Russo JE, et al. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry 2003;25:246-52. [Crossref] [PubMed]
- Shahsavand Ananloo E, Ghaeli P, Kamkar MZ, et al. Comparing the effects of fluoxetine and imipramine on total cholesterol, triglyceride, and weight in patients with major depression. Daru 2013;21:4. [Crossref] [PubMed]
- Moreira FP, Jansen K, Cardoso TA, et al. Metabolic syndrome in subjects with bipolar disorder and major depressive disorder in a current depressive episode: Population-based study: Metabolic syndrome in current depressive episode. J Psychiatr Res 2017;92:119-23. [Crossref] [PubMed]
- Karaiskos D, Tzavellas E, Ilias I, et al. Agomelatine and sertraline for the treatment of depression in type 2 diabetes mellitus. Int J Clin Pract 2013;67:257-60. [Crossref] [PubMed]
- Kesim M, Tiryaki A, Kadioglu M, et al. The effects of sertraline on blood lipids, glucose, insulin and HBA1C levels: A prospective clinical trial on depressive patients. J Res Med Sci 2011;16:1525-31. [PubMed]
- Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry 2010;71:1259-72. [Crossref] [PubMed]
- Alam MY, Jacobsen PL, Chen Y, et al. Safety, tolerability, and efficacy of vortioxetine (Lu AA21004) in major depressive disorder: results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol 2014;29:36-44. [Crossref] [PubMed]
- Hough CM, Lindqvist D, Epel ES, et al. Higher serum DHEA concentrations before and after SSRI treatment are associated with remission of major depression. Psychoneuroendocrinology 2017;77:122-30. [Crossref] [PubMed]
- Lecrubier Y. How do you define remission? Acta Psychiatr Scand Suppl 2002.7-11. [Crossref] [PubMed]
- Kuang WH, Dong ZQ, Tian LT, et al. MicroRNA-451a, microRNA-34a-5p, and microRNA-221-3p as predictors of response to antidepressant treatment. Braz J Med Biol Res 2018;51:e7212. [Crossref] [PubMed]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959;32:50-5. [Crossref] [PubMed]
- Reyes Ortega M, Soto Hernández AL, Milla Kegel JG, et al. Actualización de la Escala de Depresión del Centro de Estudios Epidemiológicos (CES-D). Estudio piloto en una muestra geriátrica mexicana. Salud Mental 2003;26:59-68.
- McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia 2010;53:66-9. [Crossref] [PubMed]
- Burkhauser RV, Cawley J. Beyond BMI: the value of more accurate measures of fatness and obesity in social science research. J Health Econ 2008;27:519-29. [Crossref] [PubMed]
- Rachdi C, Damak R, Fekih Romdhane F, et al. Impact of sertraline on weight, waist circumference and glycemic control: A prospective clinical trial on depressive diabetic type 2 patients. Prim Care Diabetes 2019;13:57-62. [Crossref] [PubMed]
- Cohen J. F Tests on Means in the Analysis of Variance and Covariance. In: Cohen J. editor. Statistical Power Analysis for the Behavioral Sciences. Abingdon-on-Thames: Routledge, 1977:273-406.
- Llorca PM, Lancon C, Brignone M, et al. Relative efficacy and tolerability of vortioxetine versus selected antidepressants by indirect comparisons of similar clinical studies. Curr Med Res Opin 2014;30:2589-606. [Crossref] [PubMed]
- Gois C, Duarte TA, Paulino S, et al. Depressive symptoms are associated with poor glycemic control among women with type 2 diabetes mellitus. BMC Res Notes 2018;11:38. [Crossref] [PubMed]
- Petrak F, Herpertz S, Albus C, et al. Cognitive Behavioral Therapy Versus Sertraline in Patients With Depression and Poorly Controlled Diabetes: The Diabetes and Depression (DAD) Study: A Randomized Controlled Multicenter Trial. Diabetes Care 2015;38:767-75. [Crossref] [PubMed]
- Nishimura A, Aritomi Y, Sasai K, et al. Randomized, double-blind, placebo-controlled 8-week trial of the efficacy, safety, and tolerability of 5, 10, and 20 mg/day vortioxetine in adults with major depressive disorder. Psychiatry Clin Neurosci 2018;72:64-72. [Crossref] [PubMed]
- de Groot M, Pinkerman B, Wagner J, et al. Depression treatment and satisfaction in a multicultural sample of type 1 and type 2 diabetic patients. Diabetes Care 2006;29:549-53. [Crossref] [PubMed]
- Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with Type 2 diabetes: a longitudinal study. Diabet Med 2009;26:622-7. [Crossref] [PubMed]
- Melin EO, Svensson R, Thunander M, et al. Gender, alexithymia and physical inactivity associated with abdominal obesity in type 1 diabetes mellitus: a cross sectional study at a secondary care hospital diabetes clinic. BMC Obes 2017;4:21. [Crossref] [PubMed]
- Rosenblat JD, Kakar R, McIntyre RS. The Cognitive Effects of Antidepressants in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int J Neuropsychopharmacol 2015. [Crossref] [PubMed]
- D'Agostino A, English CD, Rey JA. Vortioxetine (brintellix): a new serotonergic antidepressant. P T 2015;40:36-40. [PubMed]
- Bonaventure P, Kelly L, Aluisio L, et al. Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther 2007;321:690-8. [Crossref] [PubMed]
- Mork A, Pehrson A, Brennum LT, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther 2012;340:666-75. [Crossref] [PubMed]
- Schwasinger-Schmidt TE, Macaluso M. Other Antidepressants. Handb Exp Pharmacol 2019;250:325-55. [Crossref] [PubMed]
- Correll CU, Detraux J, De Lepeleire J, et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015;14:119-36. [Crossref] [PubMed]
- Subeesh V, Singh H, Maheswari E, et al. Novel adverse events of vortioxetine: A disproportionality analysis in USFDA adverse event reporting system database. Asian J Psychiatr 2017;30:152-6. [Crossref] [PubMed]
- Halford JC, Harrold JA, Lawton CL, et al. Serotonin (5-HT) drugs: effects on appetite expression and use for the treatment of obesity. Curr Drug Targets 2005;6:201-13. [Crossref] [PubMed]
- Ruetsch O, Viala A, Bardou H, et al. Psychotropic drugs induced weight gain: a review of the literature concerning epidemiological data, mechanisms and management. Encephale 2005;31:507-16. [Crossref] [PubMed]
- Perini G, Cotta Ramusino M, Sinforiani E, et al. Cognitive impairment in depression: recent advances and novel treatments. Neuropsychiatr Dis Treat 2019;15:1249-58. [Crossref] [PubMed]
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus: an abridged Cochrane review. Diabet Med 2014;31:773-86. [Crossref] [PubMed]
- Mahmood D, Akhtar M, Vohora D, et al. Comparison of antinociceptive and antidiabetic effects of sertraline and amitriptyline on streptozotocin-induced diabetic rats. Hum Exp Toxicol 2010;29:881-6. [Crossref] [PubMed]
- Raeder MB, Ferno J, Glambek M, et al. Antidepressant drugs activate SREBP and up-regulate cholesterol and fatty acid biosynthesis in human glial cells. Neurosci Lett 2006;395:185-90. [Crossref] [PubMed]
- Abosi O, Lopes S, Schmitz S, et al. Cardiometabolic effects of psychotropic medications. Horm Mol Biol Clin Investig 2018;36. [Crossref] [PubMed]