
Biomarkers in pediatric acute respiratory distress syndrome
Why is measurement of biomarkers important, particularly in the pediatric acute respiratory distress syndrome (PARDS) patient?
Biomarkers have been increasingly utilized and studied in PARDS clinical practice and research as a mechanism by which to improve overall care and health outcomes. While the translation of biomarker use into clinical practice is critical to improving overall patient health, adoption and implementation remain limited as many are still available only in the research environment (1) and most do not have the “ideal” specificity for either PARDS development or clinical outcomes related specifically to PARDS.
The future of PARDS biomarker research should include better identification of patients at-risk but not yet with PARDS as well as more accurate prediction and prognostication for those with established PARDS. Within the at-risk for PARDS population, knowledge of biomarkers indicating risk for PARDS development may help clinicians adjust monitoring and/or initiate lung protective management strategies earlier in the patients’ course in order to prevent PARDS development entirely. Similarly, better identification of those patients at risk for PARDS development may enable researchers to develop novel therapies that can be initiated earlier in the patient’s course.
Both pediatric and adult patients with established ARDS comprise a very heterogeneous group. Not surprisingly, despite 50 years of research, no pharmacologic treatment has proven effective in decreasing mortality or morbidity in adults or children with ARDS (2,3). Looking forward, the identification of combinations of bedside and plasma biomarkers may better identify which subgroups, or sub phenotypes, will best or least respond to PARDS therapies (1,4). Additionally, the development of novel point of care testing modalities will allow these biomarkers and their combinations to be best utilized in day to day patient care. The following chapter will explore each of these concepts in detail. Genomics, proteomics and metabolomics will not be covered as they appear in an alternate chapter in this journal.
What is a biomarker?
Before a clinician or researcher can identify and use biomarkers, one must first understand what exactly defines a biomarker. The term “biomarker”, a blend of “biological marker”, are objective measurements of a normal or abnormal physiologic state or response to an intervention. Biomarkers do not assess how a patient “feels, functions, or survives” (5). Thus, biomarkers are not clinical endpoints (6).
In 2015, the United States Federal Drug Administration and NIH recognized the need to develop a working definition of terms commonly used in translational and medical science, and defined a biomarker as “a characteristic that is measured as an indicator of normal biological process, pathogenic processes or responses to an exposure or intervention, including therapeutic interventions” (7). Similarly, the World Health Organization definition states a biomarker to be “any substance, structure or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease” (5).
Biomarker categories are further detailed in Table 1. The horizon for biomarker research is expansive, ranging from vital signs through basic chemistries to novel tests of blood and other tissues.
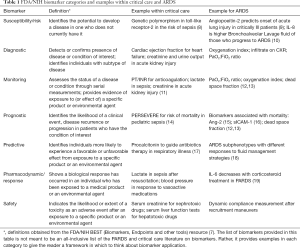
Full table
When are PARDS biomarkers best measured?
This important question is dependent on the pathophysiologic processes associated with PARDS itself and the practical considerations of when in that process the child presents to the healthcare system at the time of biomarker measurement. Traditionally, and although oversimplified, ARDS pathophysiology is divided into three phases.
The first, exudative phase, results in direct and immune-cell mediated endothelial and epithelial damage (20). Activated alveolar macrophages release proinflammatory cytokines and chemokines, which in turn recruit neutrophils and monocytes to the alveoli. Neutrophils release multiple toxic mediators, including neutrophil extracellular traps. Injury to alveolar type II cells results in surfactant inactivation and deficiency. Activation of endothelial cells and injury to the microvasculature result in barrier disruption. Taken together, this injury leads to damage of the alveolar epithelial cells, loss of barrier function and protein rich fluid accumulation.
The second, proliferative phase, encompasses the initiation of host response to restore alveolar homeostasis and repair injured tissues (20). Interstitial edema is removed through lymphatic drainage, while alveolar edema is removed via newly expressed alveolar ion channels. Vascular endothelial cell proliferation and reestablishment of tight/adherens junctions leads to restoration of endothelial and epithelial barrier functions, respectively. Neutrophils present from the initial exudative phase are phagocytosed. Finally, type II alveolar epithelial cells, responsible for surfactant production, proliferate, with some differentiating into type I cells to providing additional surface for gas exchange and fluid removal (21).
The fibrotic or final phase of ARDS leads to the development of both interstitial and intra-alveolar fibrosis. This stage, which does not occur in all patients, is associated with the need for prolonged mechanical ventilation and has been linked to an increased risk of mortality (20). Thus, associated biomarkers may be good candidates for prognostic and predictive enrichment. Damage to the basement membrane causes a lack of surfactant production and an inability to re-epithelialize. Fibroblast proliferation and differentiation leads to synthetic myofibroblasts. Additionally, accumulation of extracellular matrix results in intra-alveolar and interstitial fibrosis.
Despite the seemingly “clear” delineation of these three phases of lung injury, the pathophysiologic processes of inflammation, vascular endothelial injury, alveolar epithelial injury, fibrosis and hypercoagulability are triggered and initiated well before pediatric intensive care unit (PICU) admission and likely before initiation of invasive mechanical ventilation. Biomarker studies in children and adults, confirm evidence of lung injury and even fibrosis in the earliest days of hypoxemia and, in up to 25% of cases, before invasive mechanical ventilation has been initiated (22-26). Individual host response coupled with differences in the disease processes associated with PARDS are likely to yield different “time spent” in each phase. Patients may barely exhibit signs of certain phases yet have an overzealous response in others.
So, given a variable pathophysiologic timeline of PARDS onset and offset, when should PARDS biomarkers be measured? The 2015 Pediatric Acute Lung Injury Consensus Conference (PALICC) guidelines (27) strongly recommended “…evaluating respiratory indices and biomarkers at the onset of PARDS, within the first 24 hours of onset, as well as serial measures beyond that is indicated according to treatment and/or clinical studies”.
Importantly, as PARDS may be diagnosed in patients requiring non-invasive ventilation, researchers and clinicians alike must be wary when interpreting data from prospective, observational studies as to when sampling occurred in relation to the potential PARDS onset. For example, biomarker studies executed as “ancillary” to ongoing randomized, controlled trials are usually restricted to sample collection after the patient has been randomized into the trial. Therefore, measurements are occurring some time distant to the onset of the pathophysiologic process in question, in our consideration PARDS. Despite this important limitation, the “ancillary” study biomarker mechanism benefits greatly from the large, multi-center enrollment population than would not have occurred if the biomarker examination was attempted as an observational study alone.
In pediatrics, the multisite clinical trial, Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE; U01 HL086622) enrolled children with acute respiratory failure requiring invasive mechanical ventilation. Genetic Variation and Biomarkers in Children with Acute Lung Injury (BALI; R01HL095410), was a prospective ancillary study to RESTORE. BALI was designed to examine the association of specific plasma protein and genetic biomarkers with PARDS among prospectively enrolled children with acute respiratory failure. Although RESTORE enrolled patients with acute respiratory failure, and, therefore children before PARDS onset, BALI confirmed that approximately 90% of patients meeting PARDS criteria did so within the first day of RESTORE enrollment, thereby limiting the opportunity to find biomarkers that can be measured prior to PARDS onset and within a time frame to allow clinicians to use these measurements to initiate preventative therapies (28). Interestingly, these data corroborate with adult data from the LUNG-SAFE study wherein only 7% of adults that eventually developed ARDS did so after day 2 of acute hypoxemic respiratory failure (29).
How can PARDS biomarkers be best measured?
The ideal biomarkers are available at the bedside, quick to assay, reliable when repeated on the same subject/specimen, reproducible when measured under changing conditions or across institutions/laboratories, easily interpretable and, as least invasive as possible. Clinical biomarkers, such as oxygenation indices, satisfy most all of these criteria (30). Ventilation indices satisfy most but usually require some form of blood gas measurement. When measuring protein biomarkers, however, the best method becomes more complex.
Most PARDS biomarker research involves measurement in plasma as most PARDS patients have vascular access or laboratory studies drawn for other clinical purposes upon which the research related biomarkers are “added”. When reviewing plasma biomarker data, the reader is cautioned to note that some marker measurements require “fresh” plasma, particularly cytokine measurements, while others may be robust when measured as remnant “discard” sample (e.g., markers of endothelial injury or macrophage activation). The latter is an attractive option to families who are concerned regarding consent of their child for participation in research studies where repeated blood draws are required. This method also may be useful where “discard” blood specimens are available from the time period prior to patient consent that will still be retrievable after appropriate consent has been obtained.
The gold standard method for biomarker measurement of plasma proteins is the enzyme-linked immunosorbent assay (ELISA) (31). These measures are often made with control samples and in duplicate to assure reliability. Typically, only one marker can be measured per sample, which can be prohibitive for pediatric patients where a limited amount of plasma is available. Fluorescent bead-based Luminex techniques can measure multiple (12 to 100) analytes, usually cytokines, using a fraction of the specimen amount required for traditional ELISA assays. Nonetheless, not all Luminex assays share the same accuracy, with coefficients of variation ranging from 0% to 76% depending on the cytokine measured. Therefore, specimens should be measured alongside controls with known concentrations of the cytokines in question (32). Finally, exciting research opportunities now exist using nano- and microfluidic technology for low volume, point of care measurement of a number of inflammatory cytokines (33,34).While still in the research domain, these newest techniques show the most promise of these cytokines to be measured as part of routine patient care, the ultimate “bench to bedside” scenario.
Ideally, the most accurate assessment of PARDS biomarkers should emanate from the lungs themselves. Unfortunately, many PARDS patients are too unstable to tolerate bronchoalveolar lavage (BAL) in the most severe phases of disease (35). Further, BAL fluids are, by definition, diluted with saline in specimen acquisition (36). Many adult ARDS researchers have employed undiluted tracheal aspirate measurements, however such specimens are only attainable in the earliest phases of illness and when overwhelming amounts of pulmonary edema fluid have been generated. Others have lauded microsample probe (37,38) or mini-BAL (39-41). Exhaled breath condensate (EBC) is non-invasive and evolving in utility and reproducibility. Although paired protein measurements in EBC do not exactly correlate with that measured in BAL fluid (42), measurement of protein, pH, inflammatory cytokines, chemokines, isoprostanes, peroxides, phospholipids, adhesion and other molecules have been successful in both adults and children after transplant, with asthma, bronchpulmonary dysplasia, pulmonary hypertension etc. (43). In 2017, McNeil et al. demonstrated a technique to measure ARDS related protein biomarkers using EBC collected in the heat moisture exchanger from the exhalation limb of mechanical ventilator circuits (44). Concentrations of proteins measured were similar to those obtained in undiluted edema fluid from the same patients. This technique has not yet been applied in PARDS patients (45,46).
What PARDS Biomarkers can we measure?
The following section further augments other recently published, outstanding reviews in this field (45-47).
Vascular endothelial biomarkers
The vascular endothelium, the inner portion of the alveolar-capillary barrier, is thought to play a significant role in ARDS due to its many functions including regulation of vascular tone, inflammation and cytokine production, and coagulation (48). As such, plasma biomarkers of endothelial activity have been utilized as surrogate markers for its injury. Endothelial biomarkers including angiopoeitin-2 (Ang-2), von Willebrand Factor (vWF), endothelin, soluble E-selectin, and soluble thrombomodulin have all been associated previously with morbidity and mortality in both pediatric and adult ARDS.
Regulation of endothelial homeostasis is partially dependent on the Angiopoeitin-Tyrosine Kinases with immunoglobulin-like and EGF-like domains 2 (TIE-2) ligand-receptor system. Angiopoeitin 1 (Ang-1) and Ang-2 competitively bind the Tie-2 receptor with opposing cellular responses (49). While Ang-1 results in stabilization of the endothelium, binding of Ang-2 results in the activation and destabilization of the endothelium. As such, increased plasma levels of Ang-2 have been associated with mortality in both adult and pediatric ARDS (9,15,50-52).
vWF is produced primarily by endothelial cells and released into circulation on endothelial activation or injury. Prior studies have demonstrated vWF to be a marker of endothelial injury for patients at risk or with ARDS (53,54). And while some studies demonstrate a vWF to be predictive of ARDS development, others have not replicated this finding. Among the pediatric population, specifically, vWF is elevated in children with ARDS compared to healthy controls and associated with mortality and prolonged mechanical ventilation (24,55).
Adhesion molecules appear to play a central role in tissue damage caused by inflammatory responses because of their role in the early, transient adhesion and migration of neutrophils and monocytes to the endothelium (56,57). Among these cell surface molecules, the most important are the selectin family, which mediate rolling of neutrophils on endothelial cells with resultant endothelial cell activation, and the β2-integrin subfamily, which mediates firm adherence to the endothelium, chemotaxis, and phagocytosis. As such, several studies of adult and pediatric ARDS have associated E-selectin with both ARDS development and mortality (53,58,59).
Endothelin-1 is released from the vascular endothelium and results in cell proliferation, edema, inflammation and vasoconstriction (60,61). The limited number of studies indicates an association between Endothelin-1 and PARDS (62).
Finally, soluble thrombomodulin (sTM), a transmembrane protein highly expressed on pulmonary alveolar capillary endothelium, aids in the activation of activated protein C. While circulating levels are normally low, they increase in the presence of inflammation (63,64). sTM is elevated in both adults and children with ARDS and associated with worse clinical outcomes (65,66).
Alveolar epithelial biomarkers
Injury to pulmonary bronchial and alveolar epithelial cells are a key component of the underlying pathophysiology of ARDS. These cells are may be directly injured as in primary ARDS (e.g., pneumonia) or become subsequently injured as a result of indirect mechanism (e.g., pancreatitis). Given their integral role in both ARDS pathology and healing, biomarkers of the epithelium have been used as indicators of ARDS. While the adult literature has shown a strong association with epithelial biomarkers and ARDS, including Krebs von den Lungen-6 (KL-6), Clara (also known as club) cell secretory protein (CC16), Soluble receptor for advanced glycation end products (sRAGE), and soluble intercellular adhesion molecule 1 (sICAM-1), limited studies have been performed in children with ARDS.
sICAM-1 is a marker of epithelial integrity and essential to the maintenance of the epithelial and capillary barrier. sICAM-1 is also integral to macrophage activation and response to infection as sICAM-1 can bind to respiratory viruses, especially respiratory syncytial virus (RSV) (67). Flori and colleagues found sICAM-1 levels were significantly higher during initial days of acute lung injury in nonsurvivors and in those who required prolonged mechanical ventilation (16). Subsequent studies by others confirmed this association between sICAM-1 levels with mortality and length of mechanical ventilation (68-70). Additionally, among PARDS patients who required high frequency oscillating ventilation, elevation in sICAM-1 levels were demonstrated in children unresponsive to lung recruitment maneuvers (69).
Soluble receptor for advanced glycation end products (sRAGE), expressed by alveolar type 1 cells, can activate several signaling pathways, including NFKB and MAP-K leading to inflammation. Several adult studies demonstrated increased plasma sRAGE in patients with ARDS and have correlated levels with mortality and organ failure (71,72). Previously, studies in children with ARDS were lacking, with only one showing plasma sRAGE levels in postoperative cardiac patients is associated with the development of Acute Lung Injury (73). However, a recent study by Yehya et al., examined sRAGE levels in children with ARDS within 48 hours of PARDS onset. Elevated sRAGE was associated with both mortality and the number of non-pulmonary organ failures (74). Interestingly, sRAGE was only associated with mortality in immunocompetent patients and those with direct lung injury (74).
Krebs von den Lungen-6 (KL-6), a glycoprotein on type II pneumocytes, is released in response to cell injury or proliferation. As such, increased KL-6 levels have been associated with not only the development of ARDS in adult patients, but also worse outcomes (75-77). Briassoulis et al., compared children with ARDS to those with sepsis, traumatic brain injury or ventilated controls and found patients with ARDS had early plasma levels of KL-6 over three-fold higher than children with TBI, sepsis or ventilated controls (78). In addition, KL-6 levels were correlated with higher oxygenation index, lower PaO2:FiO2 ratio, longer length of stay and length of mechanical ventilation (78). Lastly, plasma KL-6 levels were significantly increased in non-survivors compared to survivors in those with ARDS (78).
Injury to alveolar type II cells and the resulting inability to produce surfactant is key to the underlying ARDS pathophysiology. Thus, surfactant proteins have been studied as a biomarker of acute lung injury. In children with acute lung injury, surfactant proteins A, B and D were significantly elevated in plasma samples (79). Similarly, a significant increase in surfactant protein D was observed in BAL fluid of children with acute lung injury or PARDS (79). While initial studies of exogenous surfactant use in pediatric ARDS showed promise (80,81), a 2013 randomized trial of 110 children with acute lung injury/PARDS found no significant difference in 90 day mortality, ventilator free days or ICU free days (82).
Biomarkers of dysregulated coagulation and fibrosis
Hyaline membranes, the characteristic pathology described in ARDS, are made of fibrin breakdown products and proteinaceous residua following alveolar epithelial cell injury. Indeed, abnormalities of the coagulation system are associated with morbidity and mortality in ARDS (83-86). Many of these are available as part of routine clinical practice. Among these are prolonged prothrombin time, activated thromboplastin time and thrombocytopenia. Antithrombin-III, a serine protease inhibitor, inhibits coagulation factors associated with the intrinsic pathway (Factors X, IX, XI, XII) and thrombin (Factor II). Decreased AT-III levels have been associated with increased mortality in PARDS (87). Protein C is a protein activated by the thrombin-thrombomodulin complex on endothelial cells. When activated, protein C, in combination with protein S, cleaves and inhibits factors V and VIII as well as plasminogen activation inhibitor-1, thereby both inhibiting further coagulation and enhancing fibrinolysis. Studies of plasma and intra-alveolar protein C levels in adults with ARDS indicate plasma protein C levels were significantly lower in adults with ARDS compared to control patients (88). Further, lower plasma protein C levels were associated with several important clinical outcomes including death, prolonged mechanical ventilation and increased non-pulmonary organ system failures (88).
Other plasma markers of coagulation, available to date largely in the research domain, have also been evaluated for their role and association with ARDS severity and outcome. Among these, tissue factor, tissue factor pathway inhibitor, urokinase activated protein C still have yet to be evaluated in the PARDS population. As these have been associated with pneumonia severity (89), mortality in adults (90,91), and the development/presence of ARDS (92) further investigation is warranted in children.
Survival after ARDS partly depends on the orderly reconstruction of the gas exchange apparatus. In some patients, this process leads to complete recovery of respiratory function. In contrast, other patients experience ineffective lung repair associated with excessive fibroproliferation and accumulation of fibrotic tissue that may lead to death or severe long-term pulmonary dysfunction (93,94). Identification of biomarkers that may distinguish these different phenotypes earlier in the course of disease are greatly needed.
PAI-1 functions as a primary inhibitor of tissue plasminogen activator (tPA) and urokinase, thus leading to decreased activation of plasminogen and decreased fibrinolysis (95). It is activated in the setting of inflammation and can lead to increased thrombosis or fibrin deposition. PAI-1 is synthesized primarily by endothelial cells and platelets and increases in activity in the presence of lipopolysaccharide and interleukin-1. In experimental lung injury models and in the pulmonary edema fluid of adults with ARDS, presence of fibrin degradation products and/or lung fibroblasts and activated macrophages increase PAI-1 expression and elaboration (96-98). In PARDS, elevated PAI-1 has been associated with ARDS diagnosis itself and an increased risk of mortality (66,99).
Research in animal models and adult ARDS patients confirms that the fibroproliferative response is initiated even in the earliest phases of ARDS even though the phenotypic display of this fibrosis is experienced later in the patient’s disease course. Procollagen peptides are helpful, but not sufficiently specific in identifying such patients to be used alone in this determination (100). Combining elevated procollagen peptide levels with imaging, such as high resolution computerized tomography is offering promise in improving this diagnostic and predictive yield (101).
In PARDS, matrix metalloproteinases (MMPs) have been the most commonly measured biomarkers of lung fibroproliferative response. MMPs are integral to neutrophil mediated lung inflammation but are also elaborated in fibroblasts and epithelial cells and when imbalanced with their natural inhibitors, have been associated with pulmonary fibrosis syndromes (102). MMP-8 and 9, when measured in direct tracheal aspirates of PARDS patients, are both greatly elevated in PARDS compared to non-pPARDS controls and correlate with duration of mechanical ventilation (103-105). In plasma and using latent class analysis (LCA) techniques, MMP-3, -7 and -9 together with tissue inhibitors of metalloproteinases-1 and -2 can be used to identify two distinct MMP profiles that are also associated with inflammation and endothelial injury as well as very relevant clinical outcomes including oxygenation defect, multiple organ system failures and death (105).
Inflammatory biomarkers
Pro-inflammatory cytokines are released from alveolar macrophages, neutrophils and other cells in response to injury. These cytokines, including tumor necrosis factor alpha (TNF), interleukin-1 receptor agonist (IL-1ra), Interleukin-6 (IL-6), interleukin-8 (IL-8), recruit neutrophils, monocytes and macrophages to the alveoli and activate epithelial cells and T-cells which in turn continue to sustain ongoing inflammation and injury.
While TNF has been associated with the development of and subsequent outcomes of adults with ARDS, the findings in pediatric ARDS are less clear. In one study of corticosteroids and ARDS, TNF levels were correlated with interleukin-10 (IL-10) and interleukin-1 alpha (19). Similarly, other pro-inflammatory cytokines including IL-6 have been strongly associated with ARDS severity and outcomes in adults. Again the data is less well defined in pediatrics. IL-6, however, has demonstrated usefulness as a response biomarker as it was found to decrease with corticosteroid treatment in pediatric ARDS (19). Finally, Interferon-gamma (IFN), another pro-inflammatory cytokine, as well as the IFN:IL-10 ratio was found to be significantly higher in non-survivors of pediatric ARDS (106).
IL-8, another pro-inflammatory cytokine, is elevated in in both plasma and BAL specimens in pediatric ARDS. In 2010, Todd et al. demonstrated that IL-8 levels were significantly higher in children with acute lung injury compared to controls and that this difference was maintained throughout the course of ventilation (79). More recently, Flori et al. examined plasma IL-8 levels in mechanically ventilated children (23). IL-8 levels were significantly higher in children with pediatric ARDS and independently associated with death, duration of mechanical ventilation and PICU length of stay. It was, however, not associated with pediatric ARDS development.
IL-10, the most commonly studied and important anti-inflammatory cytokine, inhibits proinflammatory cytokines, suppresses antigen presentation and enhances B cell survival. In children with ARDS, IL-10 levels at 1 week of hospitalization were associated with plateau pressure and discharge oxygen use (19).
While pro- and anti-inflammatory cytokines have been well studied in the adult ARDS and pediatric lung pathology arenas, research specific to pediatric ARDS is somewhat limited (22). Much of our knowledge relies on extrapolating from the adult and neonatal literature or studies, which include pediatric ARDS patients. As cytokines have potential to be employed as prognostic, predictive, monitoring and response biomarkers, further investigation is warranted.
PAMPS, DAMPS and extracellular vesicles (EVs)
The pathogenesis of ARDS is mediated by both pathogen-associated molecular patterns (PAMPS) and damage-associated molecular patterns (DAMPS). PAMPS, such as lipopolysaccharide, are extrinsic molecules from microorganisms. DAMPS are intracellular molecules released during injury that can then bind to other cell surfaces and activate nuclear factor kappa B translocation into the nucleus, stimulating further inflammation or possibly even by polarizing macrophages toward an immunosuppressive phenotype (107). Examples include histone, mitochondrial DNA, hyaluronic acid, double stranded DNA and the high mobility box proteins. DAMPS have been measured in the BAL fluid of cystic fibrosis and burn patients and offer promise as biomarkers in PARDS as well (108). For example, Yehya et al. have measured circulating nucleosomes in children with PARDS and found striking and independent associations with mortality and non-pulmonary organ failure and severity of oxygenation defect (74). In contrast, EVs are membranous vesicles intentionally budded off of plasma membranes that can message across cells and stimulate uncontrolled thrombosis and inflammation (109). EVs are promising new biomarkers and potential targets for precision therapies in the future for both adult and PARDS (110).
Clinical or “bedside” biomarkers
It should not be forgotten that there are a myriad of “bedside biomarkers” that have been successfully used to risk stratify PARDS patients. Again, no one bedside biomarker alone has been able to ideally predict either PARDS development, length of PARDS course, response to therapy or ultimate outcome.
Among pediatric ARDS patients, both oxygenation index (OI) and PaO2/FiO2 ratio represent prognostic and predictive biomarkers for the severity of disease (111). In fact, indices of oxygenation defect are the only ARDS biomarker for which patient monitoring and management guidelines exist. These markers have been measured in a multitude of pediatric acute lung injury studies over the last 30 years, but data utility has been limited due to small sample sizes and lack of a pediatric specific definition for PARDS (27).
The 2015 PALICC definitions of PARDS have now limited these discrepancies. Earlier clinical research indicated strong predictive ability when oxygenation defect was measured at PARDS onset (27). Using 2015 PALICC definitions, a large, prospective international study of PARDS incidence confirmed that best mortality discrimination, driven by measurement of OI or oxygenation saturation index (OSI), occurred at 6 hours after PARDS diagnosis (112). Another large, prospective cohort study confirmed both PaO2/FiO2 and OI at 6 and 24 hours provided good discrimination and calibration for death and duration of mechanical ventilation (113).
Oxygenation reflects only a portion of the physiological deficits in PARDS. Several pediatric studies have demonstrated the predictive value of CO2 exchange, and thereby lung perfusion, on outcomes just as has been seen in the adult population. Worse lung perfusion is thought to be due to dysregulated coagulation and vascular endothelial injury in the lungs of PARDS patients. Volume capnography has enabled noninvasive measurement of dead space, the alveolar component that is ventilated but not perfused and therefore not participating in gas exchange. While the Douglas Bag method of dead space determination remains the gold standard method for dead space measurement (114,115), end tidal alveolar dead space fraction (AVDSf) has also been shown to be both elevated in PARDS patients and independently predictive of mortality when measured at PARDS onset (12,13).
As our understanding of PARDS pathophysiology improves, it is important to recognize the incremental improvement in outcome prediction by measures that combine clinical “biomarkers”. Use of OSI, OI, PaO2/FiO2 or SaO2/FiO2 in addition to AVDSf has been shown to identify children with PARDS at high risk for death (116). Similarly, in a multicenter investigation of 20 clinical biomarkers, both logistic regression analyses and 10-fold cross validation modelling resulted in a parsimonious model of increasing OI and history of cancer or hematopoietic stem cell transplantation yielding the best discrimination and mortality prediction (117).
Finally, the use of chest imaging as a biomarker to aid in PARDS prediction and prognostication, whether by chest radiograph, computerized tomography, lung ultrasound and other modalities remain another active and emerging area of biomarker research (118-121).
Combining clinical indices and plasma biomarkers: identification of ARDS and PARDS sub-phenotypes
Heterogeneity in patients with ARDS likely contributes significantly to the failure of the treatments tested thus far (2). Simulation models demonstrate that heterogeneity in cohorts of patients with acute respiratory failure can significantly impact clinical trial results (3). In a negative trial, there may be no benefit for the entire cohort, while a high-risk subgroup (sub phenotype) of patients may actually benefit from the treatment. On the other hand, a ‘positive trial’ showing benefit for the entire cohort may result in harm to a subgroup of patients due to the treatment (3).
Drs. Calfee and Delucci have used LCA to identify 2 sub phenotypes in adult ARDS patients with different biomarker profiles, clinical and biological characteristics, clinical outcomes, and response to both nonpharmacologic and pharmacologic treatments (18,122,123). These findings have since been independently replicated in 4 different NHLBI-funded adult ARDS trials (123). The hyperinflammatory ARDS sub phenotype, characterized in part by higher plasma inflammatory biomarkers [IL-6, IL-8, soluble tumor necrosis factor receptor-1 (sTNFr-1), PAI-1, Ang-2, RAGE], and decreased Protein C, is associated with 20–30% higher mortality and approximately 10-day longer length of mechanical ventilation. The two sub phenotypes appear to be stable to at least 3 days after meeting ARDS criteria. While no PARDS latent class analyses exist to date, Yehya and Wong applied classification and regression tree (CART) analysis in PARDS patients. They determined age plus 3 biomarkers (C-C chemokine ligand 3, IL-8 and heat shock protein A1B) can identify children with low, intermediate and high risk of death (124). Assuredly, LCA and CART are another emerging area of PARDS biomarker research.
Conclusions
Biomarker research in PARDS is evolving and expanding rapidly. The future is bright and includes many novel biomarkers evaluable throughout all aspects of the PARDS pathophysiological process. Insights into markers specific for at risk patients as well as assessment of combinations of plasma, lung fluid and clinical biomarkers in established PARDS are likely to uncover important subphenotypes that will herald the next phases of precision medicine. Finally, technological advances in biomarker measurement will allow for small volume measurements, so integral to the pediatric population, as well as point of care assessments that will truly enable the bench to be brought to the bedside.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Wu AC, Kiley JP, Noel PJ, et al. Current Status and Future Opportunities in Lung Precision Medicine Research with a Focus on Biomarkers. An American Thoracic Society/National Heart, Lung, and Blood Institute Research Statement. Am J Respir Crit Care Med 2018;198:e116-36. [Crossref] [PubMed]
- Prescott HC, Calfee CS, Thompson BT, et al. Toward Smarter Lumping and Smarter Splitting: Rethinking Strategies for Sepsis and Acute Respiratory Distress Syndrome Clinical Trial Design. Am J Respir Crit Care Med 2016;194:147-55. [Crossref] [PubMed]
- Iwashyna TJ, Burke JF, Sussman JB, et al. Implications of Heterogeneity of Treatment Effect for Reporting and Analysis of Randomized Trials in Critical Care. Am J Respir Crit Care Med 2015;192:1045-51. [Crossref] [PubMed]
- Ware LB, Koyama T, Billheimer DD, et al. Prognostic and Pathogenetic Value of Combining Clinical and Biochemical Indices in Patients With Acute Lung Injury. Chest 2010;137:288-96. [Crossref] [PubMed]
- World Health Organization WHO International Programme on Chemical Safety. Biomarkers In Risk Assessment: Validity And Validation. Available online: https://apps.who.int/iris/handle/10665/42363
- Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS 2010;5:463-6. [Crossref] [PubMed]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring (MD): Food and Drug Administration (US), 2016.
- Gao JW, Zhang AQ, Wang X, et al. Association between the TLR2 Arg753Gln polymorphism and the risk of sepsis: a meta-analysis. Crit Care 2015;19:416. [Crossref] [PubMed]
- Agrawal A, Matthay MA, Kangelaris KN, et al. Plasma Angiopoietin-2 Predicts the Onset of Acute Lung Injury in Critically Ill Patients. Am J Respir Crit Care Med 2013;187:736-42. [Crossref] [PubMed]
- Donnelly SC, Strieter RM, Kunkel SL, et al. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 1993;341:643-7. [Crossref] [PubMed]
- Selewski DT, Cornell TT, Heung M, et al. Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensive Care Med 2014;40:1481-8. [Crossref] [PubMed]
- Bhalla AK, Belani S, Leung D, et al. Higher Dead Space Is Associated With Increased Mortality in Critically Ill Children. Crit Care Med 2015;43:2439-45. [Crossref] [PubMed]
- Yehya N, Bhalla AK, Thomas NJ, et al. Alveolar Dead Space Fraction Discriminates Mortality in Pediatric Acute Respiratory Distress Syndrome*. Pediatr Crit Care Med 2016;17:101-9. [Crossref] [PubMed]
14. Wong HR, Weiss SL, Giuliano JS, et al. The Temporal Version of the Pediatric Sepsis Biomarker Risk Model. PLoS One 2014;9:e92121-7. - Zinter MS, Spicer A, Orwoll BO, et al. Plasma angiopoietin-2 outperforms other markers of endothelial injury in prognosticating pediatric ARDS mortality. Am J Physiol Lung Cell Mol Physiol 2016;310:L224-31. [Crossref] [PubMed]
- Flori HR, Ware LB, Glidden D, et al. Early elevation of plasma soluble intercellular adhesion molecule-1 in pediatric acute lung injury identifies patients at increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med 2003;4:315-21. [Crossref] [PubMed]
- Rhee C. Using Procalcitonin to Guide Antibiotic Therapy. Open Forum Infect Dis 2016;4:ofw249. [Crossref] [PubMed]
- Famous KR, Delucchi K, Ware LB, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med 2017;195:331-8. [Crossref] [PubMed]
- Schwingshackl A, Kimura D, Rovnaghi CR, et al. Regulation of inflammatory biomarkers by intravenous methylprednisolone in pediatric ARDS patients: Results from a double-blind, placebo-controlled randomized pilot trial. Cytokine 2016;77:63-71. [Crossref] [PubMed]
- Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med 2017;377:562-72. [Crossref] [PubMed]
- Lasnier JM, Wangensteen OD, Schmitz LS, et al. Terbutaline stimulates alveolar fluid resorption in hyperoxic lung injury. J Appl Physiol 1996;81:1723-9. [Crossref] [PubMed]
- Zinter MS, Orwoll BE, Spicer AC, et al. Incorporating Inflammation into Mortality Risk in Pediatric Acute Respiratory Distress Syndrome. Crit Care Med 2017;45:858-66. [Crossref] [PubMed]
- Flori H, Sapru A, Quasney MW, et al. A prospective investigation of interleukin-8 levels in pediatric acute respiratory failure and acute respiratory distress syndrome. Crit Care 2019;23:128. [Crossref] [PubMed]
- Flori HR, Ware LB, Milet M, et al. Early elevation of plasma von Willebrand factor antigen in pediatric acute lung injury is associated with an increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med 2007;8:96-101. [Crossref] [PubMed]
- Xu Z, Wu G-M, Li Q, et al. Predictive Value of Combined LIPS and ANG-2 Level in Critically Ill Patients with ARDS Risk Factors. Mediators Inflamm 2018;2018:1739615.
- Sapru A, Curley MAQ, Brady S, et al. Elevated PAI-1 is associated with poor clinical outcomes in pediatric patients with acute lung injury. Intensive Care Med 2010;36:157-63. [Crossref] [PubMed]
- The Pediatric Acute Lung Injury Consensus Conference Group. Pediatric Acute Respiratory Distress Syndrome. Pediatr Crit Care Med 2015;16:428-39. [Crossref] [PubMed]
- Dahmer MK, Quasney MW, Sapru A, et al. Interleukin-1 Receptor Antagonist Is Associated With Pediatric Acute Respiratory Distress Syndrome and Worse Outcomes in Children With Acute Respiratory Failure. Pediatr Crit Care Med 2018;19:930-8. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. The LUNG SAFE study: a presentation of the prevalence of ARDS according to the Berlin Definition! Crit Care. Crit Care 2016;20:268. [Crossref] [PubMed]
- Bartlett JW, Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol 2008;31:466-75. [Crossref] [PubMed]
- Lequin RM. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin Chem 2005;51:2415-8. [Crossref] [PubMed]
- Djoba Siawaya JF, Roberts T, Babb C, et al. An evaluation of commercial fluorescent bead-based luminex cytokine assays. PLoS One 2008;3:e2535. [Crossref] [PubMed]
- Song Y, Chen P, Chung MT, et al. AC Electroosmosis-Enhanced Nanoplasmofluidic Detection of Ultralow-Concentration Cytokine. Nano Lett 2017;17:2374-80. [Crossref] [PubMed]
- Chang HN, Leroueil PR, Selwa K, et al. Profiling Inflammatory Responses with Microfluidic Immunoblotting. PLoS One 2013;8:e81889-8. [Crossref] [PubMed]
- Sauaia A, Moore FA, Moore EE, et al. Diagnosing pneumonia in mechanically ventilated trauma patients: endotracheal aspirate versus bronchoalveolar lavage. J Trauma 1993;35:512-7. [Crossref] [PubMed]
- Dargaville PA, South M, McDougall PN. Comparison of two methods of diagnostic lung lavage in ventilated infants with lung disease. Am J Respir Crit Care Med 1999;160:771-7. [Crossref] [PubMed]
- Singh S, Grover V, Christie L, et al. A Comparative Study of Bronchoscopic Microsample Probe versus Bronchoalveolar Lavage in Patients with Burns-Related Inhalational Injury, Acute Lung Injury and Chronic Stable Lung Disease. Respiration 2015;89:19-26. [Crossref] [PubMed]
- Ishizaka A, Watanabe M, Yamashita T, et al. New bronchoscopic microsample probe to measure the biochemical constituents in epithelial lining fluid of patients with acute respiratory distress syndrome. Crit Care Med 2001;29:896-8. [Crossref] [PubMed]
- Hendrickson CM, Abbott J, Zhuo H, et al. Higher mini-BAL total protein concentration in early ARDS predicts faster resolution of lung injury measured by more ventilator-free days. Am J Physiol Lung Cell Mol Physiol 2017;312:L579-85. [Crossref] [PubMed]
- Agrawal A, Zhuo H, Brady S, et al. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: results from two clinical trials. Am J Physiol Lung Cell Mol Physiol 2012;303:L634-9. [Crossref] [PubMed]
- Srinivasan R, Song Y, Wiener-Kronish J, et al. Plasminogen activation inhibitor concentrations in bronchoalveolar lavage fluid distinguishes ventilator-associated pneumonia from colonization in mechanically ventilated pediatric patients. Pediatr Crit Care Med 2011;12:21-7. [Crossref] [PubMed]
- Jackson AS, Sandrini A, Campbell C, et al. Comparison of Biomarkers in Exhaled Breath Condensate and Bronchoalveolar Lavage. Am J Respir Crit Care Med 2007;175:222-7. [Crossref] [PubMed]
- Yen E, Weinberger BI, Laumbach RJ, et al. Exhaled breath condensate nitrite in premature infants with bronchopulmonary dysplasia. J Neonatal Perinatal Med 2018;11:399-407. [Crossref] [PubMed]
- McNeil JB, Shaver CM, Kerchberger VE, et al. Novel Method for Noninvasive Sampling of the Distal Airspace in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2018;197:1027-35. [Crossref] [PubMed]
- Orwoll BE, Sapru A. Biomarkers in Pediatric ARDS: Future Directions. Front Pediatr 2016;4:55-15. [Crossref] [PubMed]
- Sapru A, Flori H, Quasney MW, et al. Pathobiology of acute respiratory distress syndrome. Pediatr Crit Care Med 2015;16:S6-22. [Crossref] [PubMed]
- Yehya N. Pediatric ARDS biomarkers: missing the random forest for the trees. Crit Care. Crit Care 2019;23:97. [Crossref] [PubMed]
- Goldenberg NM, Kuebler WM. Endothelial cell regulation of pulmonary vascular tone, inflammation, and coagulation. Compr Physiol 2015;5:531-59. [Crossref] [PubMed]
- Pierce RW, Giuliano JS Jr, Pober JS. Endothelial Cell Function and Dysfunction in Critically Ill Children. Pediatrics 2017. [Crossref] [PubMed]
- van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, et al. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax 2008;63:903-9. [Crossref] [PubMed]
- Gallagher DC, Parikh SM, Balonov K, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock 2008;29:656-61. [PubMed]
- Calfee CS, Gallagher D, Abbott J, et al. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med 2012;40:1731-7. [Crossref] [PubMed]
- Moss M, Gillespie MK, Ackerson L, et al. Endothelial cell activity varies in patients at risk for the adult respiratory distress syndrome. Crit Care Med 1996;24:1782-6. [Crossref] [PubMed]
- Ware LB, Conner ER, Matthay MA. von Willebrand factor antigen is an independent marker of poor outcome in patients with early acute lung injury. Crit Care Med 2001;29:2325-31. [Crossref] [PubMed]
- El Basset Abo El Ezz AA, Abd El Hafez MA, El Amrousy DM, et al. The predictive value of Von Willebrand factor antigen plasma levels in children with acute lung injury. Pediatr Pulmonol 2017;52:91-7. [Crossref] [PubMed]
- Shanley TP, Warner RL, Ward PA. The role of cytokines and adhesion molecules in the development of inflammatory injury. Mol Med Today 1995;1:40-5. [Crossref] [PubMed]
- Zimmerman GA, Albertine KH, Carveth HJ, et al. Endothelial activation in ARDS. Chest 1999;116:18S-24S. [Crossref] [PubMed]
- Okajima K, Harada N, Sakurai G, et al. Rapid assay for plasma soluble E-selectin predicts the development of acute respiratory distress syndrome in patients with systemic inflammatory response syndrome. Transl Res 2006;148:295-300. [Crossref] [PubMed]
- Al-Biltagi MA, Abo-Elezz AAE, Elshafiey RM, et al. The predictive value of soluble endothelial selectin plasma levels in children with acute lung injury. J Crit Care 2016;32:31-5. [Crossref] [PubMed]
- Comellas AP, Briva A. Role of endothelin-1 in acute lung injury. Transl Res 2009;153:263-71. [Crossref] [PubMed]
- Kowalczyk A, Kleniewska P, Kolodziejczyk M, et al. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp (Warsz) 2015;63:41-52. [Crossref] [PubMed]
- Dobyns EL, Eells PL, Griebel JL, et al. Elevated plasma endothelin-1 and cytokine levels in children with severe acute respiratory distress syndrome. J Pediatr 1999;135:246-9. [Crossref] [PubMed]
- Boehme MW, Galle P, Stremmel W. Kinetics of thrombomodulin release and endothelial cell injury by neutrophil-derived proteases and oxygen radicals. Immunology 2002;107:340-9. [Crossref] [PubMed]
- Martin FA, Murphy RP, Cummins PM. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol 2013;304:H1585-97. [Crossref] [PubMed]
- Orwoll BE, Spicer AC, Zinter MS, et al. Elevated soluble thrombomodulin is associated with organ failure and mortality in children with acute respiratory distress syndrome (ARDS): a prospective observational cohort study. Crit Care 2015;19:435. [Crossref] [PubMed]
- Sapru A, Calfee CS, Liu KD, et al. Plasma soluble thrombomodulin levels are associated with mortality in the acute respiratory distress syndrome. Intensive Care Med 2015;41:470-8. [Crossref] [PubMed]
- Mohapatra SS, Lockey RF. Respiratory syncytial virus infection: from biology to therapy: a perspective. World Allergy Organ J 2008;1:21-8. [Crossref] [PubMed]
- Al-Biltagi MA, Abo-Elezz AA, Abu-Ela KT, et al. The Prognostic Value of Soluble Intercellular Adhesion Molecule 1 Plasma Level in Children With Acute Lung Injury. J Intensive Care Med 2017;32:320-5. [Crossref] [PubMed]
- Samransamruajkit R, Jiraratanawong K, Siritantiwat S, et al. Potent inflammatory cytokine response following lung volume recruitment maneuvers with HFOV in pediatric acute respiratory distress syndrome. Asian Pac J Allergy Immunol 2012;30:197-203. [PubMed]
- Samransamruajkit R, Prapphal N, Deelodegenavong J, et al. Plasma soluble intercellular adhesion molecule-1 (sICAM-1) in pediatric ARDS during high frequency oscillatory ventilation: a predictor of mortality. Asian Pac J Allergy Immunol 2005;23:181-8. [PubMed]
- Jabaudon M, Blondonnet R, Roszyk L, et al. Soluble Receptor for Advanced Glycation End-Products Predicts Impaired Alveolar Fluid Clearance in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2015;192:191-9. [Crossref] [PubMed]
- Calfee CS, Ware LB, Eisner MD, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 2008;63:1083-9. [Crossref] [PubMed]
- Liu X, Chen Q, Shi S, et al. Plasma sRAGE enables prediction of acute lung injury after cardiac surgery in children. Crit Care 2012;16:R91. [Crossref] [PubMed]
- Yehya N, Thomas NJ, Meyer NJ, et al. Circulating markers of endothelial and alveolar epithelial dysfunction are associated with mortality in pediatric acute respiratory distress syndrome. Berlin Heidelberg: Springer, 2016:1-9.
- Nakashima T, Yokoyama A, Ohnishi H, et al. Circulating KL-6/MUC1 as an independent predictor for disseminated intravascular coagulation in acute respiratory distress syndrome. J Intern Med 2008;263:432-9. [Crossref] [PubMed]
- Nathani N, Perkins GD, Tunnicliffe W, et al. Kerbs von Lungren 6 antigen is a marker of alveolar inflammation but not of infection in patients with acute respiratory distress syndrome. Crit Care 2008;12:R12. [Crossref] [PubMed]
- Sato H, Callister ME, Mumby S, et al. KL-6 levels are elevated in plasma from patients with acute respiratory distress syndrome. Eur Respir J 2004;23:142-5. [Crossref] [PubMed]
- Briassoulis G, Mavrikiou M, Margeli A, et al. Circulating levels of KL-6 in acute respiratory distress syndrome sepsis or traumatic brain injury in critically ill children. Pediatr Pulmonol 2006;41:790-5. [Crossref] [PubMed]
- Todd DA, Marsh MJ, George A, et al. Surfactant phospholipids, surfactant proteins, and inflammatory markers during acute lung injury in children. Pediatr Crit Care Med 2010;11:82-91. [Crossref] [PubMed]
- Willson DF, Zaritsky A, Bauman LA, et al. Instillation of calf lung surfactant extract (calfactant) is beneficial in pediatric acute hypoxemic respiratory failure. Members of the Mid-Atlantic Pediatric Critical Care Network. Crit Care Med 1999;27:188-95. [Crossref] [PubMed]
- Willson DF, Jiao JH, Bauman LA, et al. Calf's lung surfactant extract in acute hypoxemic respiratory failure in children. Crit Care Med 1996;24:1316-22. [Crossref] [PubMed]
- Willson DF, Thomas NJ, Tamburro R, et al. Pediatric calfactant in acute respiratory distress syndrome trial. Pediatr Crit Care Med 2013;14:657-65. [Crossref] [PubMed]
- Wang T, Liu Z, Wang Z, et al. Thrombocytopenia is associated with acute respiratory distress syndrome mortality: an international study. PLoS One 2014;9:e94124. [Crossref] [PubMed]
- Zarbock A, Ley K. The role of platelets in acute lung injury (ALI). Front Biosci 2009;14:150-8. (Landmark Ed). [Crossref] [PubMed]
- Han YJ, Park JD, Choi JW, et al. Coagulopathy as a Prognostic Factor of Acute Lung Injury in Children. J Korean Med Sci 2012;27:1541-6. [Crossref] [PubMed]
- Bozza FA, Shah AM, Weyrich AS, et al. Amicus or adversary: platelets in lung biology, acute injury, and inflammation. Am J Respir Cell Mol Biol 2009;40:123-34. [Crossref] [PubMed]
- Lee YS, Kim S, Kang EK, et al. Antithrombin-III as an early prognostic factor in children with acute lung injury. Korean J Pediatricsm 2007;50:443-8. [Crossref]
- Matthay MA, Ware LB. Plasma protein C levels in patients with acute lung injury: prognostic significance. Crit Care Med 2004;32:S229-32. [Crossref] [PubMed]
- Wrotek A, Jackowska T, Pawlik K. Soluble urokinase plasminogen activator receptor: an indicator of pneumonia severity in children. Adv Exp Med Biol. Cham: Springer International Publishing, 2015;835:1-7.
- Jalkanen V, Yang R, Linko R, et al. SuPAR and PAI-1 in critically ill, mechanically ventilated patients. Intensive Care Med 2013;39:489-96. [Crossref] [PubMed]
- Sapru A, Liu KD, Wiemels J, et al. Association of common genetic variation in the protein C pathway genes with clinical outcomes in acute respiratory distress syndrome. Crit Care. Crit Care 2016;20:151. [Crossref] [PubMed]
- Ware LB, Fang X, Matthay MA. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol 2003;285:L514-21. [Crossref] [PubMed]
- Martin C, Papazian L, Payan MJ, et al. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest 1995;107:196-200. [Crossref] [PubMed]
- Marshall RP, Bellingan G, Webb S, et al. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med 2000;162:1783-8. [Crossref] [PubMed]
- Burnham EL, Janssen WJ, Riches DW, et al. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J 2014;43:276-85. [Crossref] [PubMed]
- Olman MA, Hagood JS, Simmons WL, et al. Fibrin fragment response elements in the plasminogen activator inhibitor gene. Chest 1999;116:118S-9S. [Crossref] [PubMed]
- Horton MR, Olman MA, Noble PW. Hyaluronan fragments induce plasminogen activator inhibitor-1 and inhibit urokinase activity in mouse alveolar macrophages: a potential mechanism for impaired fibrinolytic activity in acute lung injury. Chest 1999;116:17S. [Crossref] [PubMed]
- Hagood JS, Olman MA, Godoy JA, et al. Regulation of type I plasminogen activator inhibitor by fibrin degradation products in rat lung fibroblasts. Blood 1996;87:3749-57. [PubMed]
- Fiore-Gartland A, Panoskaltsis-Mortari A, Agan AA, et al. Cytokine Profiles of Severe Influenza Virus-Related Complications in Children. Front Immunol 2017;8:1423-12. [Crossref] [PubMed]
- Clark JG, Milberg JA, Steinberg KP, et al. Type III procollagen peptide in the adult respiratory distress syndrome. Association of increased peptide levels in bronchoalveolar lavage fluid with increased risk for death. Ann Intern Med 1995;122:17-23. [Crossref] [PubMed]
- Hamon A, Scemama U, Bourenne J, et al. Chest CT scan and alveolar procollagen III to predict lung fibroproliferation in acute respiratory distress syndrome. Ann Intensive Care 2019;9:42. [Crossref] [PubMed]
- Lanchou J, Corbel M, Tanguy M, et al. Imbalance between matrix metalloproteinases (MMP-9 and MMP-2) and tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in acute respiratory distress syndrome patients. Crit Care Med 2003;31:536-42. [Crossref] [PubMed]
- Kong MY, Li Y, Oster R, et al. Early Elevation of Matrix Metalloproteinase-8 and -9 in Pediatric ARDS Is Associated with an Increased Risk of Prolonged Mechanical Ventilation. PLoS One 2011;6:e22596-8. [Crossref] [PubMed]
- Hästbacka J, Linko R, Tervahartiala T, et al. Serum MMP-8 and TIMP-1 in Critically Ill Patients with Acute Respiratory Failure. Anesth Analg 2014;118:790-8. [Crossref] [PubMed]
- Zinter MS, Delucchi KL, Kong MY, et al. Early Plasma Matrix Metalloproteinase Profiles. A Novel Pathway in Pediatric Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2019;199:181-9. [Crossref] [PubMed]
- Phung TT, Suzuki T, Phan PH, et al. Pathogen screening and prognostic factors in children with severe ARDS of pulmonary origin. Pediatr Pulmonol 2017;52:1469-77. [Crossref] [PubMed]
- Maile R, Jones S, Pan Y, et al. Association between early airway damage-associated molecular patterns and subsequent bacterial infection in patients with inhalational and burn injury. Am J Physiol Lung Cell Mol Physiol 2015;308:L855-60. [Crossref] [PubMed]
- Fujishima S. Pathophysiology and biomarkers of acute respiratory distress syndrome. J Intensive Care 2014;2:32. [Crossref] [PubMed]
- Eppensteiner J, Davis RP, Barbas AS, et al. Immunothrombotic Activity of Damage-Associated Molecular Patterns and Extracellular Vesicles in Secondary Organ Failure Induced by Trauma and Sterile Insults. Front Immunol 2018;9:190. [Crossref] [PubMed]
- McVey MJ, Maishan M, Blokland KEC, et al. Extracellular vesicles in lung health, disease, and therapy. Am J Physiol Lung Cell Mol Physiol 2019;316:L977-89. [Crossref] [PubMed]
- Ray S, Rogers L, Pagel CR, et al. PaO2/FIO2 Ratio Derived From the SpO2/FIO2 Ratio to Improve Mortality Prediction Using the Pediatric Index of Mortality-3 Score in Transported Intensive Care Admissions*. Pediatr Crit Care Med 2017;18:e131-6. [Crossref] [PubMed]
- Khemani RG, Smith L, Lopez-Fernandez YM, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med 2019;7:115-28. [Crossref] [PubMed]
- Yehya N, Thomas NJ, Khemani RG. Risk Stratification Using Oxygenation in the First 24 Hours of Pediatric Acute Respiratory Distress Syndrome. Critical Care Medicine 2018;46:619-24. [Crossref] [PubMed]
- Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 2002;346:1281-6. [Crossref] [PubMed]
- Lum L, Saville A, Venkataraman ST. Accuracy of physiologic deadspace measurement in intubated pediatric patients using a metabolic monitor: comparison with the Douglas bag method. Crit Care Med 1998;26:760-4. [Crossref] [PubMed]
- Ghuman AK, Newth CJ, Khemani RG. The association between the end tidal alveolar dead space fraction and mortality in pediatric acute hypoxemic respiratory failure. Pediatr Crit Care Med 2012;13:11-5. [Crossref] [PubMed]
- Spicer AC, Calfee CS, Zinter MS, et al. A Simple and Robust Bedside Model for Mortality Risk in Pediatric Patients With Acute Respiratory Distress Syndrome. Pediatr Crit Care Med 2016;17:907-16. [Crossref] [PubMed]
- Warren MA, Zhao Z, Koyama T, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax 2018;73:840-6. [Crossref] [PubMed]
- Zaglam N, Jouvet P, Flechelles O, et al. Computer-aided diagnosis system for the Acute Respiratory Distress Syndrome from chest radiographs. Comput Biol Med 2014;52:41-8. [Crossref] [PubMed]
- Bass CM, Sajed DR, Adedipe AA, et al. Pulmonary ultrasound and pulse oximetry versus chest radiography and arterial blood gas analysis for the diagnosis of acute respiratory distress syndrome: a pilot study. Crit Care 2015;19:282. [Crossref] [PubMed]
- Bello G, Blanco P. Lung Ultrasonography for Assessing Lung Aeration in Acute Respiratory Distress Syndrome: A Narrative Review. J Ultrasound Med 2019;38:27-37. [Crossref] [PubMed]
- Bos LD, Schouten LR, van Vught LA, et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax 2017;72:876-83. [Crossref] [PubMed]
- Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014;2:611-20. [Crossref] [PubMed]
- Yehya N, Wong HR. Adaptation of a Biomarker-Based Sepsis Mortality Risk Stratification Tool for Pediatric Acute Respiratory Distress Syndrome. Crit Care Med 2018;46:e9-16. [Crossref] [PubMed]


