One problem two issues! Left ventricular systolic and diastolic dysfunction in aortic stenosis
Introduction
Aortic stenosis (AS) is the most common valvular lesion that affects 2-7% of the population age >65 years worldwide (1). Increased afterload caused by the stenotic valve inevitably leads to systolic and diastolic dysfunction. It is suggested that once the symptoms of left ventricular (LV) dysfunction (syncope, angina, and dyspnoea) develop, survival is reduced to 2-3 years without surgical intervention (2,3). To date, no medicinal therapy has been proven to effectively modify the natural history of patients with AS (4). Aortic valve replacement (AVR) remains the only definitive therapy for such patients (5). Reports have suggested progressive restoration of the LV structure and restoration of systolic and diastolic function (6-9) post AVR and that a patient’s long-term survival is largely dependent on this progressive LV restoration (10).
With the advancement of surgical and anaesthetic techniques, operative mortality in patients undergoing isolated AVR has significantly decreased; currently averaging 3-5% (11). Some studies have shown that in-hospital mortality is as low as 0.5% post-AVR (12), suggesting a promising decrease in acute post-operative mortality. Diastolic heart function is a reliable prognostic indicator of post-operative mortality (13). The relationship between AVR, surgical recovery and diastolic function may have a high impact on short term post-operative mortality. Here we present the most relevant and current literature on post-AVR diastolic failure and its sequel on the outcome of post AVR population.
Patho-aetiology of aortic stenosis
AS can lead to an elevated peak transaortic flow velocity (above 4 m/s), causing 70% of patients to require AVR within the next 2 years (11). The most common etiology of AS is senile calcification of a normal tri-leaflet valve and then followed by prevalence of congenital bicuspid valve (14,15). However, the literature so far remains undefined on etiology (16). Historically, studies have reported that AS is an age-related degenerative process that leads to accumulation of calcium on the leaflets, causing narrowing of the valve orifice (16-18). However, recent reports have suggested that AS could be the result of active inflammatory processes that involves biochemical, humoral and genetic factors (19,20). Though, it had also been previously hypothesised that the pathogenesis of AS might be secondary to a process similar to atherosclerosis. This hypothesis was mainly based on documentation of (I) histological similarities between the lesion of aortic stenosis and atheromatous coronary artery disease; (II) association between atherosclerotic risk factors and (III) development of aortic stenosis. AS lesion histology demonstrated active inflammation involving lipid accumulations, inflammatory cell infiltration and calcification of the base and leaflets of the cusp (5,16-22). Low-density-lipoprotein cholesterol (LDL-cholesterol) and lipoprotein A infiltrates can undergo oxidative modifications within the valve. Similar to atherosclerotic lesions, these lipoproteins then stimulate the inflammatory cascade and mineralisation (18,19).
The risk factors for AS are similar to the risk factors for atherosclerosis. As such, hypertension, dyslipidaemia, male gender, smoking, diabetes and chronic renal insufficiency (23,24) are extensively studied in this respect. It is suggested that treatment with hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, an effective treatment for atherosclerosis, may delay the progression of AS. One earlier non-randomised prospective trial demonstrated a significant reduction in rate of progression in patients treated with Rosuvastatin, a drug that reduces LDL cholesterol in the blood (18). However, the latest large scale randomised control trials (RCTs), the SEAA and the ASTRONOMER trial, reported that statins are not associated with a reduction in the progression of AS (17,25,26), despite a significant reduction of cholesterol levels and/or inflammatory markers (IL-6, C-reactive protein, soluble CD-40 ligand).
Recent studies have also suggested that the pathological expression of bone formation markers may play a role in the pathogenesis of AS. Specific markers of bone formation such as bone matrix proteins (e.g., osteopontin, osteocalcin, and bone sialoprotein) (27-30) and osteoblast transcription factor, Runx2 (27,31,32), have been found in human calcified aortic valves. Due to the valve calcification, 16-MCT scans can have high diagnostic value for the diagnosis of AS (33). One study also reported mature bone lamellar tissue found in surgically excised valves (34). There is also some evidence from retrospective analysis suggesting the beneficial effect of bisphosphonates on disease progression (35), however we are unaware of any prospective RCTs that have been performed. Needless to say, the exact aetiology of aortic stenosis has so far eluded a definitive explanation or treatment, other than by surgical intervention.
Pathophysiology of LV dysfunction in arotic stenosis
With disease progression the leaflet motion and the effective valve area becomes reduced due to valvular thickening and calcification along with increased rigidity and narrowing of the aortic orifice. The LV adapts to the increased systolic pressure required to maintain cardiac output (CO) through a hypertrophic process that involves the muscular and non-muscular components of the LV (7). Microscopically, the increase in myocardial fibre size and diameter along with interstitial fibrosis of the LV are responsible for changes in systolic and diastolic functions (9,36). Macroscopically, hypertrophy causes a decrease in stroke volume (SV), compliance, and elasticity. Even low grade aortic stenosis leads to a clinically significant decrease of stroke volume in 51% of patients (37). Systolic function of the LV, reflected by LV ejection fraction (LVEF), is maintained as long as the increased wall thickness is enough to counter the high intracavitary systolic pressure (38). This maintains a near normal LVEF, due to the proportionate decrease in SV/End Diastolic Volume (EDV), thus important in the diagnosis of LVDD. However, if the contractility of the LV is depressed or the hypertrophic processes are inadequate this leads to LV systolic dysfunction (36). On the other hand, optimal diastolic function of the LV is reliant on the compliance of the LV in diastole that allows the LV to fill from low LA pressure (39). The compensatory mechanisms described above occur in AS and lead to abnormally elevated end-diastolic filling pressures. The increased pressure in diastole is most notably due to increased LV stiffness, impaired LV compliance, increased myocardial tone, and ventricular wall thickness (9,37-39).
Diastolic relaxation, defined as the myocardium returning to its length and tension before systole, is not a passive process, but is an active, energy-dependent process involving ATP hydrolysis with actin and myosin cross-bridge release. Compliance is best described as a change in the volume of the heart over change in pressure (dV/dP), whereas stiffness is the reciprocal of compliance (dP/dV). As the atrium contracts in an effort to compensate for the lack of early diastolic filling, the LV filling pressures are increased above the normal. Since filling pressures are determined mainly by LV compliance and elasticity, when these are abnormal, the LV filling pressure then leads to diastolic dysfunction (36,39,40). Lund and associates confirmed that diastolic dysfunction starts at an earlier stage than the ejection fraction (EF) lowering for patients with aortic valve disease (41). When EF (systolic function) starts to decrease, it is probable that diastolic dysfunction has now already quite advanced, characterized by elevated end diastolic pressure and diminished diastolic filling (40,42,43). This could imply the need for early referral, i.e., before onset of fall in EF.
We now know that in the settings of AS, early phase of diastole is effected by four factors (I) the rate of relaxation; (II) elastic recoil of the ventricle; (III) chamber compliance; and (IV) left atrial pressure (44). The rate, elastic recoil, and chamber compliance, are all negatively affected by the features of a hypertrophic heart. The third phase of diastole is diastasis and is not heavily affected in DD. The fourth phase of diastole (A wave) is atrial contraction. This phase is dictated by the force of contraction of the atria and 20% of diastolic filling is attained here. In DD, the atrial contraction is increased to overcome the reduced compliance in the LV. As the fibrotic LV receives blood from atrial contraction, the pressure per volume raises above what it would in a normal heart. This creates the environment of a higher end diastolic pressure (44).
Diastolic filling abnormalities are classified by four grades: Grade 1, impaired relaxation; Grade 2, pseudo normalisation; Grade 3, reversible restrictive filling; and Grade 4, irreversible restrictive filling (44). A slower relaxation of the LV leads to a delayed opening of the mitral valve, yielding a prolonged 1st phase of diastole. This yields a reduced peak E velocity. Peak E is the measurement of the pressure gradient between the LA and LV (44). Atrial contraction then compensates for the reduced amount of blood in the LV creating an increase in the peak A velocity. E/A ratio in a patient with normal diastolic function are within the range at 0.75-1.5. However, as the DD increases in severity, the E/A ratio may decrease less than 0.75 and then increase back to normal. This is what is referred to as pseudo normalisation as mentioned previously (44). In the most severe cases of DD, the E/A ratio may increase above 1.5. Propagation velocity (Vp) is a measure of the velocity of propagating flow within the ventricle and is calculated via Doppler as the slope of early trans-mitral wavefront (44). It is typically increased in diastolic dysfunction. These parameters may be used to aid in the diagnosis of abnormal diastolic function in AS.
Diagnosis of diastolic dysfunction in aortic stenosis
There is some level of difficulty when it comes to diagnosing DD. There are many ways to measure the changes that occur during diastole, mainly due to the large amount of information available in Doppler Echocardiography (DE). As per the ESC and ACC/AHA guidelines for assessing LVEF, comprehensive DE is the single most clinically useful diagnostic tool (45). The diagnosis of DD is three-fold. It cannot be made without some evidence of congestive heart failure (46), normal ejection fraction (above 50%), and evidence of abnormal ventricular diastole.
Based on the ASE guidelines (47) Mahmood and coworkers (48) proposed an algorithm for routine approach to diagnose and assess DD. The authors addressed their proposed scheme within the framework of the guidelines and based on the diagnosis of a specific abnormality, i.e., impaired relaxation or decreased compliance; peak mitral annular velocities; indices of LV filling pattern corroborated with LA size.
The normal ranges of values for diagnosis of DD are not well established either (49). Kitzman et al. demonstrated that an important diagnostic characteristic of DD, as determined by echo, was the maintenance of a normal LV ejection fraction (LVEF) (50). The mean for those with DD was a LVEF of 60% as compared to 31% in those with systolic heart failure. This is accounted for by the hypertrophy of the heart proportionally lowering the volume of blood stored in the LV. This affects both variables of the EF, making it an important differentiation factor from systolic heart dysfunction. When LVDD is present, atrial contraction propels blood into the LV at increased velocity, accounting for increased A wave and decreased E/A ratio. This means that less blood is transferred into the heart during early diastole due to a decreased time for relaxation and decreased compliance. The A peak is then increased due to the compensation provided by atrial contraction late in diastole. One of the major limitations of Doppler echocardiography is in evaluating trans-mitral flow patterns. Great variation in Doppler measurements are seen as these flow patterns are dependent on various factors including heart rate (51), preload, afterload (52), and LV systolic function (53). To overcome this issue, newer methods for diagnosis of LVDD, such as flow propagation velocity (Vp) via M-mode Doppler and Tissue Doppler imaging (TDI), have recently received more attention. There is evidence that Vp is less sensitive to variations in heart rate and preload (53), and the ratio of peak E velocity to Vp is a more reliable marker of the LA pressure. Thereby, Vp may serve as a more reliable, independent maker for LVDD and LV filling pressure (52). This has been supported in patients undergoing AVR, with one study showing Vp to be the superior echocardiographic marker for LVDD in terms of prognostic value and reliability. Tissue Doppler imaging (TDI), which measures the high intensity, low velocity echo seen in the myocardium, was recently developed. It is unique in its ability to obtain the velocity of diastolic wall motion (e') and its timing, which enables assessment of regional relaxation abnormalities and their global effect on ventricular relaxation and filling dynamics (42). In a study comparing E'/E values with conductance catheters, it was found that the elevation of pressure needed for diastolic filling can be directly related to an elevation in the speed of diastolic wall motion (E') (54). Parameters that have been shown to be highly associated with LV diastolic function and are widely used for the evaluation of LVDD include mean pulmonary wedge pressure (PWP) or mean left atrial pressure, LV end-diastolic pressure (LVEDP), time constant of LV relaxation, and LV passive-stiffness constant (42). This is of little use clinically due to the risks involved in direct cardiac catheterization of the left ventricle.
Correlation between LV geometry and LV diastolic function
Eccentric hypertrophy is a major contributing factor to DD. Hypertrophy is classified into two types, concentric and eccentric (54). In concentric hypertrophy, new sarcomeres are added in-parallel to already existing sarcomere, compensating for the pressure overload experienced by the myocardium (54). This is seen in subjects that participate in high-intensity strength training. On the other hand, eccentric hypertrophy is where volume overload results in ventricular dilation (54). Instead of new sarcomeres being added in parallel, as seen in concentric hypertrophy, they are added in-series to existing sarcomeres (54). Therefore pressure overload is associated with concentric hypertrophy, whereas volume overload is associated with eccentric hypertrophy (54). Impaired relaxation is seen in both concentric and eccentric hypertrophies (8), but chamber stiffness is seen to increase only in concentric LV hypertrophy (37).
Patients with AS were found to have typical concentric LV hypertrophy (LVH) before AVR, and their LV mass index (LVMI)/LV end-diastolic volume index (LVEDVI) ratio were much higher than the control subjects without AS (54). Post-AVR, it was found that the LVMI/LVEDVI ratio and pressure overload decreased to values closer to the control subjects (55). In a past study, Kumpuris et al. described hypertrophy as the LV wall thickness increased in proportion to LV radius; LV systolic wall stress was preserved and thereby prevented permanent heart failure and dilation (56). In another study, it was determined that AS patients with a relatively lower LV wall thickness and eccentric hypertrophy resulted in systolic dysfunction and other symptoms associated with heart failure (56). However, factors such as the presence of supernormal EF and “disproportionally high” LV wall thickness were associated with a significantly high perioperative risk of morbidity and mortality in patients with AS before AVR (46,52).
Diastolic function and LV remodelling post AVR
Adaptive changes seen in patients with AS are the end products of increased trans-valvular gradient and afterload (36,40,41). Though it has been shown that AVR leads to immediate hemodynamic improvement (46), the regression of the LV structure, LV systolic and diastolic function have also been shown to continue for decades after AVR (8,9).
A recent study (57) from Cleveland group suggested that the preoperative symptoms were not suggestive of the degree of LV hypertrophy or diastolic dysfunction. The authors reported that the patients with severe LV hypertrophy (≥180 g/m2) had reduced long-term survival compared with the patients with a LVMI of less than 96 g/m2 at 5 years (73% vs. 81%) and 10 years (45% vs. 56%), despite successful AVR. Patients with a severely enlarged left atrium (≥5.0-cm diameter) had substantially reduced long-term survival compared to patients with a diameter of less than 3.55 cm at 5 (61% vs. 85%) and 10 (28% vs. 62%) years after AVR (P=0.006) (57).
As previously described, LV hypertrophy in patients with AS is associated with increased muscle fibre diameter and interstitial fibrosis (35,36). Early after AVR, there is significant regression of LV hypertrophy (6-9), which is mainly due to the regression of muscular tissue while the total amount of non-muscular tissue (fibrous tissue) of the LV remains unchanged (9). Parallel to the relative increase in fibrous content, a deterioration of LV diastolic function early after AVR (40) in the settings of LVDD is reported and even increased LV mass might not be effective in normalizing wall stress and maintaining LV function (7). The decrease in cardiac output might result in lower than expected transvalvular gradients, with either a preserved or decreased LV ejection fraction (LVEF) (8). This underestimation of AS leads to a late indication for AVR with a negative effect on the prognosis. Milano et al., recent work demonstrated that in patients with severe AS, a reduced LV function and increased LV diameters were strongly related to the amount of myocardial fibrosis, which significantly affects long-term survival after AVR. Patients with a higher grade of myocardial fibrosis had a significantly lower freedom from cardiac death at 10 years with congestive heart failure (58).
Villari and colleagues reported that the regression of LV interstitial fibrosis and reversal of LV diastolic function is in fact a two-step process-with relative increase early after AVR and a relative reduction in LV fibrous content after 4 to 5 years (8,9). Post-AVR, it was found that patients with mild or moderate diastolic dysfunction benefited more than patients with a more advanced case (13). Evidence indicates that patients experience significant reduction in LV mass index (LVMI) and LVMI/LVEDVI (LV end-diastolic volume index) ratio, improvement in diastolic filling, and normal LV mass is achieved in up to two-thirds of the patients 10 years post-AVR (6-8,56). However, patients with aortic regurgitation (AR) that underwent AVR showed an increase in the LVMI/LVEDVI ratio and deterioration of diastolic filling, relative to patients with AS (56). On the other hand, reversal of diastolic function and reduction of the fibrous content of the LV appears to be a slow process and continue long after AVR.
Earlier studies indicate normalisation of LV diastolic function does occurs in the long-term (52), however, a later study suggests otherwise (8). Gjertsson et al. showed evidence that despite a decrease in LVMI, development of moderate to severe diastolic dysfunction persisted 10 years post-AVR (8).
In patients undergoing AVR, the exposure of the heart to ischemia and reperfusion (IR) and subsequent events (e.g., high cytosolic Ca2+, low ATP concentrations, and free radical generation) (59), may lead to post-operative systoic dysfunction but could include worsening of impaired diastolic function. If LV dysfunction is severe, these patients may experience difficulties in weaning from cardiopulmonary bypass (CBP), thus requiring positive inotropic drugs (PIDs) and mechanical circulatory support such as intra-aortic balloon pump (60). This ‘myocardial-stunning phenomenon’ is usually transient, but it may lead to post-operative complications and death (61). In patients undergoing cardiac surgery, postoperative LV dysfunction has long been identified as a major cause of mortality (62). In isolated AVR, postoperative LV dysfunction has been shown to be associated with an almost fivefold increase in mortality, and subsequently prolonged ICU stay, and increased morbidity in these patients (63). Grunenfelder et al., reported patients that underwent AVR and coronary artery bypass saw a regression in the LV mass to a lesser extent than patients with AVR alone. The study reported that ejection fraction did not improve, which suggests CAD negatively impacted myocardial recovery postoperatively (64). Even with normalisation of LV mass and EF, diastolic dysfunction still was evident during exercise and seemed to persist for several years post-AVR (55).
Management of LV dysfunction post AVR
Weaning from CPB after cardiac surgery is generally guided by hemodynamic measurements and trans-oesophageal echocardiographic (TOE) assessment. There is a lack of generally accepted criteria of defining post-operative LV dysfunction. From the studies analysed in this review, the diagnostic criteria for post-CPB LV dysfunction is largely based on the need of inotropic support (dobutamine, epinephrine, norepinephrine and milrinone) in the presence of low MAP (<60 mmHg) and with persistent, new or worsening LV functional impairment (assessed by TOE) (65).
Classically, patients who developed LV dysfunction after cardiac surgery are treated with a combination of inotropic agents such as dobutamine, epinephrine, norepinephrine, dopamine, and phosphodiesterase III inhibitors (PD III inhibitors, e.g., milrinone, and enoximone) (66), and/or with the use of an intra-aortic balloon pump (IABP) (67).
Today, reported studies have determined that the IABP has a well-established role in the management of LV dysfunction after cardiac surgery (67). There is clear evidence that IABP improves cardiac index (CI) irrespective of timing of intervention, while earlier intervention is associated with superior outcome (67). These beneficial effects are achieved through augmentation of diastolic pressure, thereby increasing coronary perfusion; and reduction of ventricular afterload, that increases stroke volume and cardiac output. The rate of major complications in IABP is low. Previously, data from the 2001 Benchmark Registry reported that the rate of major complications from IABP was 2.6% (7,61). We also reported from our recent study that complications were at lower rate of approximately 1% (58).
Most inotropic agents achieve their positive inotropic effect via increasing myocardial concentration of cAMP, thereby up-regulating the activity of protein kinase A (PKA) and in turn increasing the concentration of Ca2+ in cardiac myocytes (68). Although the inotropic agents appear to improve clinical symptoms and hemodynamic they are associated with adverse reactions such as arrhythmias, cell death, and increased long-term mortality (69). Newer agents such as levosimendan, a pyridazinone-dinitrite calcium sensitizer, have recently received more interest due to the lack of adverse effects associated with other inotropic agents. Levosimendan increases cardiac contractility without increasing myocardial oxygen demand through mechanisms different to classic inotropic agents (68). Studies have shown that levosimendan improves CI, irrespective of timing of intervention in cardiac surgery patients with LV dysfunction and low cardiac output syndrome (LCOS) (68,69). We previously suggested that both levosimendan and IABP improve cardiac function in cardiac surgery patients with LCOS, but more data is needed to clarify whether levosimendan or the IABP is superior in terms of morbidity, mortality, cardiac function, or cost-benefit (61).
Predictors of postoperative LV dysfunction
Several independent risk factors have been identified as significant predictors of post-operative LV dysfunction in patients undergoing AVR. These can generally be separated into two broad categories i.e. preoperative, and perioperative factors. The preoperative factors include; hypertension, LV systolic dysfunction, LV diastolic dysfunction (LVDD), previous history of heart failure and recent myocardial infarction (MI). The perioperative factors include; patient-prosthesis mismatches (PPM), aortic cross clamp time and intraoperative MI (66). In contrast to coronary artery bypass surgery it was reported by a recent study that there is slightly less association between preoperative and perioperative ischemia and postoperative LV dysfunction in patients undergoing AVR (62).
A large number of studies have focused on prosthesis type, prosthesis size and clinical outcome. Currently, evidence suggests that mild to moderate mismatch between patient body surface area and the prosthesis is a significant predictor for postoperative hemodynamics, and regression of LV mass (70). However, there is still conflicting data on its impact on patient outcome and survival. Several studies further suggest that patient factors, such as age, hypertension and LV systolic function are more significant determinants of LV dysfunction than mild to moderate PPM (70-73). Markers of systolic dysfunction, such as decreased LVEF, have been extensively studied to help guide the use of inotropic support during pre- and post-AVR. The information gained has been incorporated in scoring algorithms for predicting perioperative risk (72,73). Interestingly, LVDD may coexist and even precede LV systolic dysfunction (68,69). It is suggested that deterioration of LV compliance and relaxation in LVDD, with preservation of systolic function, as evidenced by prolonged relaxation and increased ventricular wall stiffness, are seen in almost 90% of patients with AS (74). It is also reported that LVDD can precede the LV systolic dysfunction in patients with AS, thus deducing that LVDD may be an early marker for the detection of abnormal LV function in these patients (59-61).
Post-AVR acute diastolic dysfunction
As previously described, the hypertrophy, increased fibrosis, and decreased elasticity of the LV leads to an interesting result immediately after a successful AVR. The myocardium, which was been compensated for the stenotic valve, had lower elasticity (due to fibrosis and myocyte hypertrophy) and an increased LV wall thickness, but less need for high LV pressures. With time we see long-term regression of the fibrosis, but in the short term we see a relative increase in interstitial fibrosis as compared to the decrease in hypertrophic myocytes (15). This, along with the myocardial stunning that accompanies cardiac surgery, increases the likelihood of short-term complications from diastolic dysfunction (DD) than that of an AS patient by accentuating the decreased elasticity of the heart. The acute loss of myocytes due to stunning as well as the thinning of the ventricular wall leads to a decrease in LV relaxation time. Decreased relaxation time in the setting of reduced elasticity and increased fibrosis could lead to acute DD that is difficult to manage. It is important to mention here that atrial fibrillation in such acute DD, further worsens this situation and increases postoperative mortality (41,71,75-77).
The negative impact of cross-clamp period and the cardiopulmonary bypass pump circuit post-AVR may significantly affect the diastolic function as well, especially in patients with more severe diastolic dysfunction preoperatively. Grade 3 (reversible) DD may be converted to Grade 4 (irreversible) DD after the AVR procedure in these susceptible patients (Figure 1). Immediately post-AVR with rapid decline in afterload compared to preop, LVEDV may become as close to being emptied in a hyperdynamic hypertrophic LV. The LV now depends much more on diastolic filling in order to keep up with low blood pressure and CO. However, in these severely DD patients, the relative LV fibrotic tissue as compared to functional myocardium layer may be increased due to damage from the cardiopulmonary bypass effect, especially involving the susceptible marginal zone, which in turn causes ineffective diastolic filling. Added fluid to these patients may cause an increase in passive pulmonary hypertension (most of these patients already have some components of pulmonary hypertension) which may interdependently shifts the interventricular septum into the LV, and further decreases the output from the right heart to the left heart to fill the emptied LV. Pharmacologic vasoactive drugs are used to help the BP in ICU, but their effects can be detrimental by causing the already thick, stiff ventricle more impaired in relaxation and less compliant. Therefore, management of immediate postop-AVR for severe DD patients shall be very challenging with worsened prognosis (Figure 2).

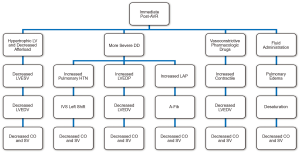
Progression of diastolic dysfunction to heart failure
Heart failure still remains to be a common cause of cardiovascular disease; they are seen in patients that have a normal or abnormal left ventricular ejection fraction (LVEF). However, heart failure (HF) patients with a normal LVEF differ substantially than HF patients with an abnormal LVEF in many ways, such as demographics, ventricular functioning and remodeling, and pathophysiological mechanisms (71-74,78). Patients with abnormal ventricular relaxation may be due to factors such as pressure overload and/or ischemia, thereby leading to impairment in relaxation during exertion (78). Patients in this initial stage of diastolic dysfunction may remain asymptomatic for many years. However, as the disease progresses, LV filling pressures and pulmonary pressure during exercise are elevated. With further progression, hypertrophy and MI results in increased myocardial stiffness, leading to elevated left atrial pressure and size, atrial fibrillation, decreased cardiac output (CO), reduced exercise tolerance, and signs of congestive heart failure (CHF) (78).
Since diastole is the period of the cardiac cycle where myocardium relaxes and lengthens in order to return to its unstressed length and force, DD may develop due to failure of the myocardium to relax or prolongation of myocardial contraction (79). Zile et al. defines diastolic heart failure as symptoms and signs of heart failure, with a preserved EF and abnormal diastolic dysfunction. Diastolic abnormalities may be caused by ventricular relaxation impairment and/or increased myocardial ventricular wall stiffness (79).
Best evidence so far
Here, we have also systemically analysed the current body of evidence on the predictive value of preoperative LV diastolic dysfunction on likelihood of left ventricle dysfunction after AVR. Medline 1966-Aug 2012 using the OVID interface [exp Cardiac surgical Procedures/OR open heart surgery.mp OR OR cardiac surgery.mp OR CABG.mp OR] AND [exp dystolic dysfunction/OR congestive heart failure.mp OR noncompliant left ventricle] AND Maximally sensitive RCT filter LIMIT to Human studies. The above search was then repeated in the Cochrane Central Register of Controlled Trials. Twenty seven papers were found from Medline and 7 papers were found in the Cochrane Central Register of Controlled Trials and were selected as providing the best evidence. Of note if two papers were found investigating the same combination of treatment, only the best paper was included. These papers are presented in Table 1.
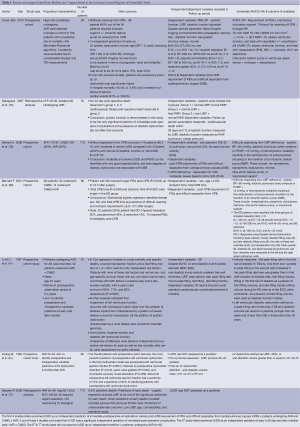
Full table
Conclusions
In summary, abnormal diastolic filling patterns are frequently observed during AVR. They are more common in patients with altered LV function. Diastolic dysfunction presents before CPB can be associated with an increased risk of postoperative complications and vasoactive support in the postoperative ICU settings. The data review presented here supports the view that evaluation of diastolic function should be routinely part of the echocardiographic assessment of patients undergoing AVR. The review provides evidence that diastolic dysfunction, as defined by Vp <40 cm/s, in addition to advanced age and prolonged ischemic time, identifies patients at risk of LV dysfunction after valvular aortic surgery. Clinicians should anticipate a greater impact of perioperative transesophageal echocardiograms when weaning from bypass in treating any syatolic dysfunction with inotropes or IABP and in ICU to identify high-risk cardiac patients while improving fluid and inotropic/lusitropic drug treatments. The association of preoperative diastolic dysfunction with adverse cardiac outcome begs the question as to whether trials of specific perioperative strategies to improve LV relaxation and filling patterns should be considered in patients undergoing AVR.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Otto CM, Lind BK, Kitzman DW, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341:142-7. [PubMed]
- Sprigings DC, Forfar JC. How should we manage symptomatic aortic stenosis in the patient who is 80 or older? Br Heart J 1995;74:481-4. [PubMed]
- Iivanainen AM, Lindroos M, Tilvis R, et al. Natural history of aortic valve stenosis of varying severity in the elderly. Am J Cardiol 1996;78:97-101. [PubMed]
- Nathaniel S, Saligram S, Innasimuthu AL. Aortic stenosis: An update. World J Cardiol 2010;2:135-9. [PubMed]
- Ghaisas NK, Foley JB, O’Briain DS, et al. Adhesion molecules in nonrheumatic aortic valve disease: endothelial expression, serum levels and effects of valve replacement. J Am Coll Cardiol 2000;36:2257-62. [PubMed]
- Gelsomino S, Frassani R, Morocutti G, et al. Time course of left ventricular remodeling after stentless aortic valve replacement. Am Heart J 2001;142:556-62. [PubMed]
- Lund O, Erlandsen M. Changes in left ventricular function and mass during serial investigations after valve replacement for aortic stenosis. J Heart Valve Dis 2000;9:583-93. [PubMed]
- Gjertsson P, Caidahl K, Bech-Hanssen O. Left ventricular diastolic dysfunction late after aortic valve replacement in patients with aortic stenosis. Am J Cardiol 2005;96:722-7. [PubMed]
- Villari B, Vassalli G, Betocchi S, et al. Normalization of left ventricular nonuniformity late after valve replacement for aortic stenosis. Am J Cardiol 1996;78:66-71. [PubMed]
- Vahanian A, Otto CM. Risk stratification of patients with aortic stenosis. Eur Heart J 2010;31:416-23. [PubMed]
- Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006;48:e1-148. [PubMed]
- Malaisrie SC, McCarthy PM, McGee EC, et al. Contemporary perioperative results of isolated aortic valve replacement for aortic stenosis. Ann Thorac Surg 2010;89:751-6. [PubMed]
- Cayli M, Kanadaşi M, Akpinar O, et al. Diastolic function predicts outcome after aortic valve replacement in patients with chronic severe aortic regurgitation. Clin Cardiol 2009;32:E19-23. [PubMed]
- Elahi M, Asopa S, Khan J. The right choice of prosthesis for patients undergoing aortic valve surgery: searching the truth. Acute Card Care 2007;9:77-81. [PubMed]
- Chambers J. Aortic stenosis. BMJ 2005;330:801-2. [PubMed]
- Mohler ER 3rd, Chawla MK, Chang AW, et al. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis 1999;8:254-60. [PubMed]
- Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005;352:2389-97. [PubMed]
- Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007;49:554-61. [PubMed]
- O’Brien KD, Reichenbach DD, Marcovina SM, et al. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler Thromb Vasc Biol 1996;16:523-32. [PubMed]
- Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol 1999;19:1218-22. [PubMed]
- Wallby L, Janerot-Sjöberg B, Steffensen T, et al. T lymphocyte infiltration in non-rheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspid aortic valves. Heart 2002;88:348-51. [PubMed]
- Rajamannan NM, Subramaniam M, Rickard D, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 2003;107:2181-4. [PubMed]
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630-4. [PubMed]
- Pohle K, Mäffert R, Ropers D, et al. Progression of aortic valve calcification: association with coronary atherosclerosis and cardiovascular risk factors. Circulation 2001;104:1927-32. [PubMed]
- Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343-56. [PubMed]
- Chan KL, Teo K, Dumesnil JG, et al. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010;121:306-14. [PubMed]
- Caira FC, Stock SR, Gleason TG, et al. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol 2006;47:1707-12. [PubMed]
- Kaden JJ, Bickelhaupt S, Grobholz R, et al. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis 2004;13:560-6. [PubMed]
- Kaden JJ, Bickelhaupt S, Grobholz R, et al. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol 2004;36:57-66. [PubMed]
- Mathieu P, Voisine P, Pépin A, et al. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis 2005;14:353-7. [PubMed]
- Rajamannan NM, Subramaniam M, Caira F, et al. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation 2005;112:I229-34. [PubMed]
- Shao JS, Cheng SL, Pingsterhaus JM, et al. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest 2005;115:1210-20. [PubMed]
- Koos R, Mahnken AH, Sinha AM, et al. Aortic valve calcification as a marker for aortic stenosis severity: assessment on 16-MDCT. AJR Am J Roentgenol 2004;183:1813-8. [PubMed]
- Demer LL. A skeleton in the atherosclerosis closet. Circulation 1995;92:2029-32. [PubMed]
- Innasimuthu AL, Katz WE. Effect of bisphosphonates on the progression of degenerative aortic stenosis. Echocardiography 2011;28:1-7. [PubMed]
- Villari B, Campbell SE, Hess OM, et al. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol 1993;22:1477-84. [PubMed]
- Jander N, Minners J, Holme I, et al. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation 2011;123:887-95. [PubMed]
- Rajamannan NM, Otto CM. Targeted therapy to prevent progression of calcific aortic stenosis. Circulation 2004;110:1180-2. [PubMed]
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165-93. [PubMed]
- Brutsaert DL, Sys SU, Gillebert TC. Diastolic failure: pathophysiology and therapeutic implications. J Am Coll Cardiol 1993;22:318-25. [PubMed]
- Lund O, Flø C, Jensen FT, et al. Left ventricular systolic and diastolic function in aortic stenosis. Prognostic value after valve replacement and underlying mechanisms. Eur Heart J 1997;18:1977-87. [PubMed]
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107-33. [PubMed]
- Leite-Moreira AF. Current perspectives in diastolic dysfunction and diastolic heart failure. Heart 2006;92:712-8. [PubMed]
- Rakowski H, Carasso S. Quantifying diastolic function in hypertrophic cardiomyopathy: the ongoing search for the holy grail. Circulation 2007;116:2662-5. [PubMed]
- Arques S, Roux E, Luccioni R. Current clinical applications of spectral tissue Doppler echocardiography (E/E’ ratio) as a noninvasive surrogate for left ventricular diastolic pressures in the diagnosis of heart failure with preserved left ventricular systolic function. Cardiovasc Ultrasound 2007;5:16. [PubMed]
- Satpathy C, Mishra TK, Satpathy R, et al. Diagnosis and management of diastolic dysfunction and heart failure. Am Fam Physician 2006;73:841-6. [PubMed]
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107-33. [PubMed]
- Mahmood F, Jainandunsing J, Matyal R. A practical approach to echocardiographic assessment of perioperative diastolic dysfunction. J Cardiothorac Vasc Anesth 2012;26:1115-23. [PubMed]
- Maurer MS, Spevack D, Burkhoff D, et al. Diastolic dysfunction: can it be diagnosed by Doppler echocardiography? J Am Coll Cardiol 2004;44:1543-9. [PubMed]
- Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 2002;288:2144-50. [PubMed]
- Appleton CP. Influence of incremental changes in heart rate on mitral flow velocity: assessment in lightly sedated, conscious dogs. J Am Coll Cardiol 1991;17:227-36. [PubMed]
- van der Maaten JM, de Vries AJ, Henning RH, et al. Effects of preoperative treatment with diltiazem on diastolic ventricular function after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2001;15:710-6. [PubMed]
- Kessler KM. Diastolic heart failure. Diagnosis and management. Hosp Pract (Off Ed) 1989;24:137-41, 146-8, 158-60 passim. [PubMed]
- Kasner M, Westermann D, Steendijk P, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation 2007;116:637-47. [PubMed]
- Mihl C, Dassen WR, Kuipers H. Cardiac remodelling: concentric versus eccentric hypertrophy in strength and endurance athletes. Neth Heart J 2008;16:129-33. [PubMed]
- Lamb HJ, Beyerbacht HP, de Roos A, et al. Left ventricular remodeling early after aortic valve replacement: differential effects on diastolic function in aortic valve stenosis and aortic regurgitation. J Am Coll Cardiol 2002;40:2182-8. [PubMed]
- Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 2014;147:362-369. [PubMed]
- Milano AD, Faggian G, Dodonov M, et al. Prognostic value of myocardial fibrosis in patients with severe aortic valve stenosis. J Thorac Cardiovasc Surg 2012;144:830-7. [PubMed]
- Elahi MM, Matata BM. Myocardial protection against ischemia-reperfusion injury: novel approaches in maintaining homeostatic stability in blood. Recent Pat Cardiovasc Drug Discov 2006;1:291-305. [PubMed]
- Elahi MM, Lam J, Asopa S, et al. Levosimendan versus an intra-aortic balloon pump in adult cardiac surgery patients with low cardiac output. J Cardiothorac Vasc Anesth 2011;25:1154-62. [PubMed]
- Elahi MM, Chetty GK, Kirke R, et al. Complications related to intra-aortic balloon pump in cardiac surgery: a decade later. Eur J Vasc Endovasc Surg 2005;29:591-4. [PubMed]
- Feneck RO, Sherry KM, Withington PS, et al. Comparison of the hemodynamic effects of milrinone with dobutamine in patients after cardiac surgery. J Cardiothorac Vasc Anesth 2001;15:306-15. [PubMed]
- Vánky F, Håkanson E, Maros T, et al. Different characteristics of postoperative heart failure after surgery for aortic stenosis and coronary disease. Scand Cardiovasc J 2004;38:152-8. [PubMed]
- Grünenfelder J, Kilb I, Plass A, et al. Impact of coronary disease after aortic valve replacement. Asian Cardiovasc Thorac Ann 2009;17:248-52. [PubMed]
- Licker M, Cikirikcioglu M, Inan C, et al. Preoperative diastolic function predicts the onset of left ventricular dysfunction following aortic valve replacement in high-risk patients with aortic stenosis. Crit Care 2010;14:R101. [PubMed]
- Ravishankar C, Tabbutt S, Wernovsky G. Critical care in cardiovascular medicine. Curr Opin Pediatr 2003;15:443-53. [PubMed]
- Cohen M, Urban P, Christenson JT, et al. Intra-aortic balloon counterpulsation in US and non-US centres: results of the Benchmark Registry. Eur Heart J 2003;24:1763-70. [PubMed]
- McBride BF, White CM. Levosimendan: implications for clinicians. J Clin Pharmacol 2003;43:1071-81. [PubMed]
- Rooke GA, Feigl EO. Work as a correlate of canine left ventricular oxygen consumption, and the problem of catecholamine oxygen wasting. Circ Res 1982;50:273-86. [PubMed]
- Germing A, Lindstaedt M, Holt S, et al. Patient-prosthesis mismatch and left ventricular remodelling after implantation of Shelhigh SuperStentless aortic valve prostheses. J Cardiovasc Surg (Torino) 2008;49:539-43. [PubMed]
- Elahi M, Usmaan K. The bioprosthesis type and size influence the postoperative incidence of permanent pacemaker implantation in patients undergoing aortic valve surgery. J Interv Card Electrophysiol 2006;15:113-8. [PubMed]
- Wendt D, Osswald B, Thielmann M, et al. The EuroSCORE - still helpful in patients undergoing isolated aortic valve replacement? Interact Cardiovasc Thorac Surg 2010;10:239-44. [PubMed]
- Wendt D, Osswald BR, Kayser K, et al. Society of Thoracic Surgeons score is superior to the EuroSCORE determining mortality in high risk patients undergoing isolated aortic valve replacement. Ann Thorac Surg 2009;88:468-74; discussion 474-5. [PubMed]
- Kim YJ, Sohn DW. Mitral annulus velocity in the estimation of left ventricular filling pressure: prospective study in 200 patients. J Am Soc Echocardiogr 2000;13:980-5. [PubMed]
- Nakagawa D, Suwa M, Ito T, et al. Postoperative outcome in aortic stenosis with diastolic heart failure compared to one with depressed systolic function. Int Heart J 2007;48:79-86. [PubMed]
- Denault AY, Couture P, Buithieu J, et al. Left and right ventricular diastolic dysfunction as predictors of difficult separation from cardiopulmonary bypass. Can J Anaesth 2006;53:1020-9. [PubMed]
- Stassano P, Di Tommaso L, Vitale DF, et al. Aortic valve replacement and coronary artery surgery: determinants affecting early and long-term results. Thorac Cardiovasc Surg 2006;54:521-7. [PubMed]
- Fraser CD Jr, McKenzie ED, Cooley DA. Tetralogy of Fallot: surgical management individualized to the patient. Ann Thorac Surg 2001;71:1556-61; discussion 1561-3. [PubMed]
- Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 2002;105:1387-93. [PubMed]
- Bernard F, Denault A, Babin D, et al. Diastolic dysfunction is predictive of difficult weaning from cardiopulmonary bypass. Anesth Analg 2001;92:291-8. [PubMed]
- Hwang MH, Hammermeister KE, Oprian C, et al. Preoperative identification of patients likely to have left ventricular dysfunction after aortic valve replacement. Participants in the Veterans Administration Cooperative Study on Valvular Heart Disease. Circulation 1989;80:I65-76. [PubMed]


