
Nutrition determines outcome after severe burns
Burns is one of the few human pathologies for which nutrition therapy has repeatedly been shown to contribute to both survival and recovery (1). This strong impact was again confirmed by Guo et al. in a study published in the May issue of the British Journal of Nutrition (2). Their amazing observation is particularly important due to severity of the burn injuries included in their large homogeneous cohort: the median burn size was 95% of body surface area (BSA), with 88% BSA being 3rd degree burns, while all patients suffered inhalation injury. Of note, a major burn is defined as an injury to more than 20% BSA in adults: in the present study, the entirety of the cohort qualifies for the appellation of “massive” burn injury.
Since the 70s, complex tracer studies, endocrine and immune investigations have been conducted to understand the metabolic turmoil that is triggered by burns (1). Despite identification of important components of this complex response, hypermetabolism is still not well understood in its entirety (3). The accompanying massive protein catabolism and important weight and lean body mass losses are associated with poor outcome. Major efforts have been made to modulate and attenuate the hypermetabolic response and to adapt to the nutritional needs of these very special patients, who have been the first to develop into chronic critical illness, a problem that has been only recently been recognized in non-burn (4). Burns is the only acute pathology for which pharmacological manipulation of the metabolism belongs to recommended strategies (5): the use of propranolol to attenuate the sympathetic burst which contributes to protein catabolism, and of oxandrolone to stimulate anabolism is evidence based (1).
A weakness of many burn studies is the small size of the cohorts and their lack of homogeneity regarding burn size, age, and etiology, which render comparisons between studies very difficult. Compared with previous studies, Guo et al.’s cohort is very homogeneous (2): 90 of the 100 patients were burned more than 70% BSA and were young (36 years). The majority of patients were admitted in the context of mass casualty (blast in a factory in Kunshan, Jiangsu, China): they were treated in several intensive care units (ICU) of the same county which adds to the quality of their achievement. The treatment included classical burn resuscitation and nutritional management according to the ESPEN burn guidelines (5). Among the specific strategies that are considered in burns, the addition of glutamine to enteral feeding was used in 72 patients, and omega-3 fatty acids were used in a third of patients. Both these actions are associated with reduction of infectious complications in the context of major burns (5,6), and may have contributed to the good survival results. The 28-day mortality was only 11%, and in hospital mortality was 45%, with 42 patients dying of septic shock. A 55% survival rate in massive burns is remarkably elevated.
Importantly, there was nearly no difference upon admission between survivors and non-survivors, particularly regarding burn and illness severity. The only 3 variables to differ significantly in non-survivors were enteral feeding intolerance, a protein deficit (delivery <0.8 g/kg/day), and an energy deficit (delivery <20 kcal/kg/day).
Several specificities of burn nutrition are addressed in Guo et al.’s observation:
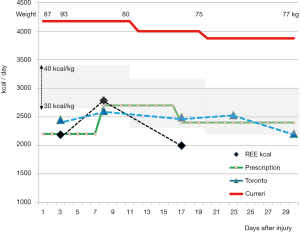
- Energy needs: due to extensive tissue repair, the energy, glucose and proteins requirements are higher compared to non-burn conditions. The increase in metabolic rate remains the most elevated among pathologies requiring ICU treatment. Indirect calorimetry (IC) is the only tool enabling appreciation of the magnitude and the evolution of the energy expenditure (EE) in individual patients. Typically the EE increases during the first 2–3 weeks and abates progressively over the next months, the magnitude of the increase being proportional to the burned surface. But IC devices are not yet largely available. The Toronto burn center was able to derive a predictive equation from multiple IC studies (7). This can replace IC in ICUs where this tool is not available: the equation integrates the impact of feeding on EE and a time factor that enables calculating the dynamic evolution of the energy targets. Figure 1 shows how well the Toronto equation fits with the measured EE, but it also shows that modern management reduces EE compared to the 1970s when the Curreri equation was developed. IC is available in our own center, but we use the Toronto equation as backup when technical issues preclude the realization of IC or if the patient is extubated. In a cohort of 240 patients admitted to our ICU, aged 43 years with much less severe burns (25% BSA as a mean), the impact of insufficient energy delivery on outcome was also observed (8). The authors calculated a tolerance cut-off for underfeeding: they found that more than two weeks underfeeding were deleterious for recovery. Patients with EN providing less than 30% of energy needs had significantly higher 28 day and in-hospital mortality than patients with EN providing more than 30% of caloric (P=0.001). Multiple regression analysis showed that low energy provision and septic shock were independent risk factors for the 28-day prognosis (P<0.05). As for any biologic responses, there is a U-shaped response curve for energy intake. Zusman et al. showed on the basis of 5012 IC studies in 1,375 non-burn critically ill patients (9), that there is an optimal ratio of energy delivery to measured EE, and that mortality increases with energy deliveries below 70% or over 110%. The same applies to critically ill burn patients, and while overfeeding is technically very difficult to achieve with EN in burns due to their high needs, overfeeding may occur particularly if the old predictive equations are applied and achieved with help of parenteral nutrition.
- Protein needs: after major burns and based on stable isotope studies, the protein needs have been shown to be about 2 g/kg in adults (up to 3 g/kg in children) (1,5). Guo et al. were able to show that a protein provision below 0.8 g/kg/day was associated with higher mortality. In addition, burns are characterized by a specific higher need for glutamine with a level A of evidence (10): this amino acid is the most important nitrogen shuttle in the body and is involved in multiple metabolic pathways such as immunity, anabolism and glucose control (11). The higher needs for glutamine in burns are among others explained by large cutaneous losses (12). Additional glutamine was provided to the majority of patients.
- Timing of nutrition: the time of gradual start of EN matters, and should be within ideally the first 24 hours, as it participates in the resuscitation of the gut by maintaining intestinal perfusion. In this observation, EN could be initiated with a median time of 1 day from injury in 67 patients, which is an amazing achievement in the context of mass casualty. The mean interval from injury to initiation of feeding for all patients was 2.4±1.1 days. Parenteral nutrition (PN) was the first feeding strategy in 22 patients. Only 32 patients developed EN intolerance. Enteral feeding intolerance occurs rather frequently affecting about 35% of patients particularly during septic episodes as shown in a study with less severe burn injuries (about 40% BSA) (13). Timing also matters in less severe burns: Vicic et al. showed in 101 patients burned >20% BSA, that feeding via a nasojejunal tube within 4 hours compared to standard meals was associated with less weight loss, less drop in albumin and lower C-reactive protein values and infection rates (14). This confirms the importance of EN as part of resuscitation, and of prevention of infectious complications.
- Route of feeding: in these severely burned patients, postpyloric feeding was more efficient than gastric feeding in terms of better EN tolerance and lower need for PN energy supplementation. Post-pyloric feeding resulted in an about 400 kcal/day, and nearly 20 g/day protein larger delivery (P=0.034 and 0.026 respectively). Majority of patients needed PN supplementation at some stage to cover their energy requirement, or due to EN feeding intolerance.
- Monitoring nutrition therapy: the authors monitored feeding: by doing so one can see the frequent and significant difference between prescription and delivery of feeds (15). Each physician was made responsible of reporting detailed management: the patients received an average of about 70% of prescribed caloric and protein dose. This study once more shows the importance of monitoring the nutritional intervention (16). The condition for success is to verify daily the adequacy of delivery. While not delivering the correct dose of antibiotics is considered a fault, lack of precisions is widely tolerated for nutrition therapy although it contributes to poor outcome. A Canadian study with the evocative title “what the dietitian prescribes is not what the burn patient gets” confirmed in 90 patients (mean burns 28% BSA) how difficult it is to cover the needs by the enteral route (15). The main reason for the discrepancy between prescription and delivery was the long duration of EN interruption of 9±3 hours per day: the causes were surgery (24%) planned extubation (7%), feed intolerance (11%), tube malfunction (2%), bedside procedures (2%), and dressing changes (3%). The median caloric deficit ranged between 172 and 930 kcal/day.
This study compares and reinforces the results of the available international literature. A study investigating international nutrition practices in burns was conducted that included 90 mechanically ventilated burn patients (945 study days): 15 countries were represented, majority of centers being Canadian, Australian and US. The severity of burn injuries was not specified, but mortality was 21%. The energy goals were determined by equations in the majority of centers, and EN was the preferred feeding route. The authors showed that worldwide burn patients develop substantial energy and protein deficits, receiving about 70% of the prescribed value. Mortality increases with growing deficit: the odds ratios were 1.10 per 100 kcal/day energy deficit, and 1.16 per 10 g/day protein (17).
Of course the study was observational and not randomized. An easy criticism is to say that we don’t know if nutrition was the hen, or the egg of outcome. Was severity of injury the reason for the worse feeding or did worse feeding cause bad outcome? The absence of difference in severity between survivors and non-survivors is an answer in favor of nutrition being the driver of the difference. This study confirms other smaller studies in less severely burned patients with strong data.
Do these results apply to non-burn critically ill patients? Probably yes, even if the energy and protein needs are lower in other categories of patients. But in those patients starting the ICU journey in poor nutritional condition (18), underfeeding will worsen outcome as shown in a cohort of chronic critically ill. Guo et al.’s results have a potential wider impact than just burns.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Rodriguez NA, Jeschke M, Williams F, et al. Nutrition in burns: Galveston contributions. JPEN J Parenter Enteral Nutr 2011;35:704-14. [Crossref] [PubMed]
- Guo F, Zhou H, Wu J, et al. A prospective observation on nutrition support in adult patients with severe burns. Br J Nutr 2019;121:974-81. [Crossref] [PubMed]
- Jeschke MG. Postburn Hypermetabolism: Past, Present, and Future. J Burn Care Res 2016;37:86-96. [Crossref] [PubMed]
- Loss SH, Nunes D, Franzosi O, et al. Chronic critical illness: are we saving patients or creating victims? Rev Bras Ter Intensiva 2017;29:87-95. [Crossref] [PubMed]
- Rousseau AF, Losser M, Ichai C, et al. ESPEN endorsed recommendations: Nutritional therapy in major burns. Clin Nutr 2013;32:497-502. [Crossref] [PubMed]
- Tihista S, Echhavarria E. Effect of omega 3 polyunsaturated fatty acids derived from fish oil in major burn patients: A prospective randomized controlled pilot trial. Clin Nutr 2018;37:107-12. [Crossref] [PubMed]
- Allard JP, Pichard C, Hoshino E, et al. Validation of a new formula for calculating energy requirements of burn patients. JPEN J Parenter Enteral Nutr 1990;14:115-8. [Crossref] [PubMed]
- Pantet O, Stoecklin P, Vernay A, et al. Impact of decreasing energy intakes in major burn patients: a 15 year retropective cohort study. Clin Nutr 2017;36:818-24. [Crossref] [PubMed]
- Zusman O, Theilla M, Cohen J, et al. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care 2016;20:367. [Crossref] [PubMed]
- Singer P, Reintam-Blaser A, Berger M, et al. ESPEN Guidelines: Nutrition in the ICU. Clin Nutr 2019;38:48-79. [Crossref] [PubMed]
- Wischmeyer PE, Dhaliwal R, McCall M, et al. Parenteral glutamine supplementation in critical illness: a systematic review. Crit Care 2014;18:R76. [Crossref] [PubMed]
- Gonzalez MR, Fleuchot B, Lauciello L, et al. Effect of human burn wound exudate on Pseudomonas aeruginosa virulence. mSphere 2016;1:e00111-15. [Crossref] [PubMed]
- Lavrentieva A, Kontakiotis T, Bitzani M. Enteral nutrition intolerance in critically ill septic burn patients. J Burn Care Res 2014;35:313-8. [Crossref] [PubMed]
- Vicic VK, Radman M, Kovacic V. Early initiation of enteral nutrition improves outcomes in burn disease. Asia Pac J Clin Nutr 2013;22:543-7. [PubMed]
- Sudenis T, Hall K, Cartotto R. Enteral nutrition: what the dietitian prescribes is not what the burn patient gets! J Burn Care Res 2015;36:297-305. [Crossref] [PubMed]
- Berger MM, Reintam-Blaser A, Calder P, et al. Monitoring nutrition in the ICU Clin Nutr 2019;38:584-93. [Crossref] [PubMed]
- Czapran A, Headdon W, Deane A, et al. International observational study of nutritional support in mechanically ventilated patients following burn injury. Burns 2015;41:510-8. [Crossref] [PubMed]
- Viana MV, Pantet O, Bagnoud G, et al. Metabolic and Nutritional characteristics of long-stay critically ill patients. J Clin Med 2019;8:985. [Crossref] [PubMed]


