
Decoding the secreted inflammatory response of primary human hepatocytes to hypoxic stress in vitro
Introduction
Hepatic ischemia and hypoxic stress are major factors affecting liver cells in relevant clinical settings such as trauma/hemorrhagic shock (T/HS) (1), ischemia/reperfusion injury (2), and liver transplantation (3). The liver is a critical, multi-functional organ that has a significant role in inflammation and innate immunity, processes that involve and are controlled by different cell types including hepatocytes (HC), Kupffer cells, and other non-parenchymal cells. In the normal adult murine liver, HC represent the majority of parenchymal cells and approximately 70–85% of liver volume (4). Numerous studies, mostly using primary murine HC and mouse cell lines, have been carried out to study the inflammatory and stress response of hepatocytes in vitro (5). We have described previously our systems biology approach to characterize the inflammatory response to stress in the liver: for example, with the use of combined experimental and computational analyses, we identified the chemokine Monocyte Chemoattractant Protein-1 (MCP-1/CCL2) as a main driver of the response of mouse hepatocytes and as a biomarker for organ damage in clinical settings of trauma and hemorrhagic shock (T/HS) (6). More recently, we showed that under hypoxic stress both mouse HC and hepatic stellate cells (HSC) release a comparable pattern of inflammatory mediators in vitro, suggesting that the hepatocyte secretory machine might be either more restricted at baseline or disrupted following stress (7). Furthermore, we showed the presence of more complex autocrine inflammatory networks in HSC as compared to HC, which supports the notion that these two cell types not only coexist but also provide a microenvironment for each other in which HSC play the “messenger” role as opposed more of a “responder” role for HC (7).
The role of inflammatory networks formed by cytokines and chemokines released by isolated primary human HC in vitro has not been investigated extensively, however. In the present study, we assessed the secreted, protein level chemokine and cytokine responses of primary human HC in response to hypoxic stress. We employed Dynamic Network Analysis (DyNA) (6,8) to discern and compare the interconnections among mediators of inflammation mediators over defined ranges of time in hypoxia vs. control. We have hypothesized previously that, as a given stress response program evolves over time, dynamic networks of inflammatory pathways lead to the evolution of a core set of inflammatory pathways, which in turn can be discerned via Principal Component Analysis (PCA) (7,9). As a refinement of this technique, we have developed a variant called Time-interval PCA (TI-PCA), which we have used recently to help define the spatiotemporal dynamics of acute inflammation in vivo (10). Our findings suggest that distinct dynamic response networks are evoked in HC in response to hypoxia as compared to cells cultured under normoxic conditions. These insights, combined with those obtained from primary mouse HC by multiple groups, increase our understanding of this important and multi-functional cell type.
Methods
Materials
Williams Medium E, penicillin, streptomycin, L-glutamine, and HEPES were purchased from Invitrogen (Carlsbad, CA). Insulin (Humulin®) was purchased from Eli Lilly (Indianapolis, IN), and calf serum was obtained from HyClone Laboratories (Logan, UT). Tissue culture dishes were from Corning Glass Works (Corning, NY). Unless indicated otherwise, all other chemicals and proteins were purchased from Sigma-Aldrich (St. Louis, MO).
Human hepatocyte isolation and culture
This study was carried out following the recommendations of the National Institutes of Health (NIH). The protocol was approved by the Institutional Review Board of the University of Pittsburgh (IRB No. 0610103). Freshly isolated human hepatocytes obtained from the tumor free portions of the liver during partial hepatectomy from patients with various primary liver or metastatic tumors were generously provided by Eric Hall (Director, Research Registry) from CellzDirect, Inc. (Durham, NC). Cells were delivered in cold preservation medium (designed to keep them viable at 4 °C) in multi-well plates with a collagen type I substratum and Matrigel® overlay as follows: 1.5 million cells/well in 6-well plates or 0.75 million cells/well in 12-well plates. Upon arrival, the preservation medium was removed and replaced by fresh William’s E medium supplemented with penicillin/streptomycin, dexamethasone, ITS, L-Glutamine and HEPES as per the provider’s specifications. After overnight incubation at 37 °C, the old medium was replaced and the cells were further incubated under hypoxic conditions for 1, 3, 6, 24, and 48 h as previously described (6,7). Hypoxic conditions were obtained by placing the cells into a modular incubator chamber (Billups-Rothenburg, Del Mar, CA) flushed with a hypoxic gas mixture containing 1% O2, 5% CO2 and 94% N2. Hepatocytes incubated under normoxic conditions (21% O2) served as control. At the end of each experiment the cell culture supernatants were collected and stored at −80 °C until analysis. Total protein isolation and determination was done using the BCA protein assay kit from Pierce (Rockford, IL) with bovine serum albumin as standard as previously described (11). All data were normalized as pg/mg total protein.
Analysis of inflammatory mediators
Human inflammatory mediators were measured using a Luminex™ 100 IS apparatus (Luminex, Austin, TX) and the Human 25-plex® Luminex™ bead kit (BioSource-Invitrogen, San Diego, CA) as per manufacturer’s specifications. The antibody bead kit included: eotaxin, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), interleukin -1 receptor antagonist (IL-1RA), interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-13, IL-15, IL-17A, interferon-γ-inducible protein 10 (IP-10/CXCL10), monocyte chemoattractant protein-1 (MCP-1/CCL2), Monokine induced by interferon-γ (MIG/CXCL9), macrophage inflammatory protein-1α (MIP-1α/CCL3), MIP-1β (CCL4), and tumor necrosis factor-α (TNF-α).
Statistical and computational analyses
We applied a number of statistical and data-driven modeling techniques aimed at discovering principal drivers, interconnected inflammatory networks, and potential key regulatory nodes of inflammation in HC exposed to cellular stress. We applied these methods in a stepwise manner following our concept of the way cells, including HC, respond to inflammatory stimuli (7,9).
- Two-Way Analysis of Variance (ANOVA) was carried out to analyze the changes in inflammatory mediators in hypoxia vs. normoxia (control) using SigmaPlot (Systat Software, San Jose, CA).
- Dynamic Bayesian network (DBN) inference was carried out to define the most likely single network structure that best characterizes the dynamic interactions among systemic inflammatory mediators across all time points, in the process suggesting likely feedback structures that define central nodes. The networks might also suggest possible mechanisms by which progression of the inflammatory response differs within a given experimental group. In this analysis, time courses of unprocessed inflammatory mediator measurements from each experimental condition were used as input for a DBN inference algorithm, implemented in MATLAB® essentially as described previously (12).
- Dynamic Network Analysis (DyNA) was carried out to define the central inflammatory network nodes as a function of time and experimental condition using our previously published algorithm (6,8,12). Connections, defined as the numbers of trajectories of inflammatory mediators that move in parallel, were created if the Pearson correlation coefficient between any two nodes (inflammatory mediators) at the same time interval was greater or equal to a threshold of 0.95, as indicated. The “network complexity” for each experimental condition was calculated using the following formula: Sum (N1 + N2 +…+ Nn)/n−1, where N represents the number of connections for each mediator and n is the total number of mediators analyzed. The total number of connections represents the sum of the number of connections for each mediator in a given experimental group.
- Time-Interval Principal Component Analysis (TI-PCA) was carried out as described recently (10), in order to identify those inflammatory mediators that contributed to the top 25% variance of the inflammatory response in human hepatocytes cultured under hypoxic or normoxic (control) conditions over four consecutive time periods (1–3, 3–6, 6–24, and 24–48 h) using MATLAB and Statistics Toolbox Release R2014b (The MathWorks, Inc., Natick, MA) (8).
Results
Patient demographics and clinical data
Primary human HC were isolated from normal liver tissue during partial hepatectomy for various cancers and subsequently cultured under hypoxic stress. The overall demographics and relevant clinical data of the eleven donors are shown in Table 1.
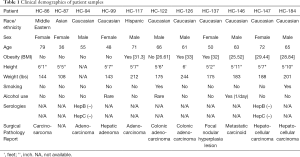
Full table
Differential trajectories of inflammatory mediators in primary human hepatocytes in response to hypoxic stress in vitro
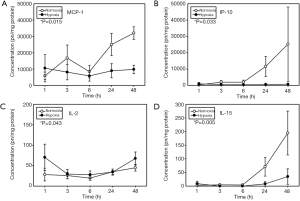
To assess the response to hypoxia, primary human HC were exposed to 1% O2 for 1–48 h, and 21 inflammatory mediators (cytokines and chemokines) were measured in the supernatant. Hepatocytes exposed to normoxic (21% O2) conditions served as control. Comparison of time-dependent changes in hypoxia vs. control showed only four mediators that exhibited significant changes in hypoxic cell cultures as compared to control: MCP-1, IP-10, IL-2 and IL-15 (Figure 1). The time-dependent release of all inflammatory mediators is shown in http://fp.amegroups.cn/cms/atm.2019.07.09-1.pdf.
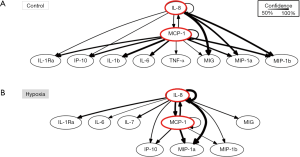
Dynamic bayesian network (DBN) inference suggests a common signature in primary human HC cultured under both hypoxic and normoxic condition
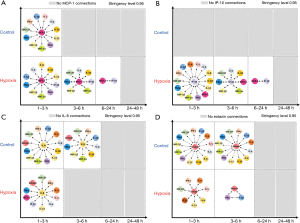
An essential aspect of dynamic networks is the manifestation of mediator feedback, which can be inferred algorithmically (9,13). We have suggested that an early signature of a cellular response to stress can be inferred via analysis of dynamic networks of inflammatory mediators (7,9). Similar to our previous studies in pediatric acute liver failure, trauma and sepsis (12,14,15), we utilized DBN inference to define putative feedback structures among the measured inflammatory mediators, in order to discern any differential regulatory architectures in hypoxic HC vs. normoxic controls (Figure 2). Though data were separated by experimental group before analysis by DBN inference, the algorithm did not make assumptions about the network connectivity in any group. Both under control (Figure 2A) and hypoxia (Figure 2B), DBN inference suggested a network regulated by mutual interactions between MCP-1 and IL-8, each of which affects its own expression. In both experimental groups, the core motif of MCP-1 and IL-8 was inferred to affect the downstream production of the chemokines IP-10, MIG, MIP-1α and MIP-1β. Key inferred differences between the two dynamic networks were the presence of TNF-α and IL-1β as output nodes in control HC (Figure 2A), vs. IL-7 in hypoxic HC (Figure 2B).
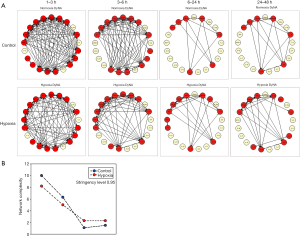
Analysis of dynamic networks of inflammatory mediators: Distinct inflammatory connectivity differentiates the response of human HC to hypoxic stress in vitro
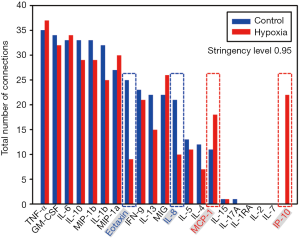
We next sought to characterize, in a more granular fashion, the time evolution of dynamic inflammatory networks of primary human HC exposed to hypoxia using Dynamic Network Analysis (DyNA). This analysis over defined ranges of time (1–3, 3–6, 6–24 and 24–48 h) initially revealed that there was a large number of network connections (Figure 3A) among the mediators released by both normoxic and hypoxic cells, but that the number of connections decreased in concert with one another within 6–12 h of culture (Figure 3A,B). However, a quantification of the total number of connections for each inflammatory mediator revealed significant differences in the connectivity of some mediators: MCP-1 and IP-10 (hypoxia > control) and Eotaxin and IL-8 (control > hypoxia) (Figure 4). We next focused on the specific connectivity of those mediators during each time interval. A detailed analysis of the MCP-1 (Figure 5A) and IP-10 (Figure 5B) connectivity revealed that while there appeared to be a marked decrease in the number of connections for both mediators over time, MCP-1 and IP-10 were more connected to myriad inflammatory mediators in hypoxic cells at 1–24 h, with no connections at all for IP-10 in control cells. Interestingly, the most persistent connection was that between MCP-1 and IP-10 (Figure 5A,B). In contrast, analysis of the IL-8 (Figure 5C) and Eotaxin (Figure 5D) connectivity showed the opposite effect: both mediators appeared to be more connected to various other inflammatory mediators in normoxic control as compared to hypoxic cells.
In addition to DyNA, we sought to use Time-Interval PCA (TI-PCA) to gain further insights into the secreted inflammatory stress responses of primary human HC (Figure 6). In support of this approach, Eotaxin was inferred as a core mediator of the early inflammatory response of HC under control normoxic conditions, along with responses involving the chemokine MIG; the cytokines IL-4, IL-6, IL-7, IL-10, IL-15, and IL-17A; and the IL-1β inhibitor IL-1RA (Figure 6A). Interestingly, IL-1β was the dominant cytokine characterizing the inflammatory response of hypoxic HC, along with IL-15 and IL-10 (and lesser contributions from other mediators, including IL-1RA, at distinct time intervals.

Discussion
The main goal of our study was to determine if combining in vitro and in silico studies could identify main hepatic inflammatory mediators in primary human HC cultured under hypoxia, a factor relevant to the pathophysiology of a number of inflammatory conditions such as ischemia/reperfusion, T/HS, and liver transplantation. We used a similar approach as in our previous studies characterizing the response of hypoxic primary mouse HC (6,7). First, the analysis of the time courses showed only four mediators that exhibited significant changes in hypoxic cell cultures as compared to control: MCP-1, IP-10, IL-15 and IL-2. Furthermore, DBN showed that in both experimental groups the two primary networks are driven by a core motif of MCP-1 and IL-8, which were inferred to affect the downstream production of IP-10, MIP-1α and MI-1β. Interestingly, we previously found a similar role for MCP-1, KC (the functional mouse homologue of human IL-8), and IP-10 in isolated primary mouse HC (6), suggesting a central role for chemokines such as MCP-1 and IL-8 in the cellular stress as a function of O2 in isolated primary human HC as well. This response does not appear to be specific to liver cells, since chemokine release, and in particular of MCP-1 and IL-8, has appears to be critical for resident human dermal fibroblasts to mediate chemotaxis of leucocytes in response to hypoxia (16). While DBN showed a common signature for both hypoxic and normoxic cells, DyNA not only showed that HC make the same suit of inflammatory mediators but despite an overlap in connectivity for some inflammatory mediators, MCP-1, IP-10, Eotaxin and IL-18 connectivity could differentiate hypoxic cells from control, again supporting that chemokines are central components of the dynamic, multi-dimensional response of human HC to cell stress in vitro.
A large number of studies, mostly using primary murine HC and mouse cell lines, have been carried out to study the inflammatory and stress response of hepatocytes in vitro. In this regard, activated Kupffer cells can regulate the release a number of cytokines and chemokines by both rodent (17) and human HC (18). Using combined experimental and computational analyses we have identified previously the chemokine MCP-1 as a principal driver of the response of mouse HC, and, by extension, as a biomarker for organ injury in T/HS (6). More recently, we have shown in vitro that both mouse HC and HSC release a repertoire of cytokines and chemokines as a response to hypoxic stress (7). The hypoxia-induced release of many inflammatory mediators is regulated by (or related to) various signaling pathways, in particular the hypoxia-inducible factor-1 (HIF-1) system, but it is not completely understood how oxygen-dependent and independent activation/inactivation of the HIF pathway acts both in HC in vitro and in the context of various chronic liver injuries (19). In this regard, we have reported previously that livers from ischemic mice demonstrated only a modest increase in HIF-1α protein as compared to resting livers from control animals; and that this expression was not statistically different from sham controls (20). In primary human HC, however, the number of studies is limited, and most of the work has been performed using HepG2 cells or liver cancer cells such as hepatocellular carcinoma (21). Of relevance to the present study is a report using multiple protein state measurements from isolated primary human hepatocytes from two human donors and HepG2 liver cancer cells cultured in the presence of growth factors or inflammatory mediators (TNF-α, IL-1α, and LPS) that separated primary from transformed hepatocytes with respect to TLR-4 signaling and NF-κB-dependent secretion of inflammatory mediators. Stimulated human HC, but not HepG2 cells, were found to secrete a “signature set” of cytokines including MCP-1, MIP-1, MIP-1β, KC, IL-6 and IP-10 (22).
There are some technical and applicability limitations to this study that cannot be overlooked when extrapolating these results to in vivo conditions. First, the relatively small number of experiments resulting from the limited availability of primary human liver cells. Furthermore, as discussed in our previous study (7), nearly all tissue culture experiments involving liver cells, such as the control conditions described herein are routinely performed under relatively hyperoxic conditions that may not replicate in vivo physiology (23). Notably, the complex oxygen gradient in the liver in vivo is very difficult to reproduce employing isolated cells in vitro, as is the identification and characterization of dynamic networks of inflammation. In addition, it is unknown if both the composition and complexity of those inflammatory networks in hepatocytes change in the presence of other liver cells. We also recognize that the primary HC used in our study are from different surgical cancer patients, so they might differ in many ways (including differences in lifestyle, genetic backgrounds and production and release of inflammatory mediators) from cell populations derived from healthy livers. Lastly, we note that the source livers were likely subjected to operative trauma, anesthesia, as well as potentially rough handling by surgeons and lab personnel as they were processed for HC isolation, which is likely to affect the secretory status of the cell monolayers in vitro.
Conclusions
In summary, our statistical and computational analyses suggest the presence of a core, conserved set of responses to stress in HC derived from surgical cancer patients, combined with distinct inflammatory sub-networks characteristic of hypoxic stress. The relevance of these findings in vivo warrants further investigation but may point to a prolongation of certain chemokine responses, specifically those centered on MCP-1 and IP-10.
Acknowledgments
Funding: This work was supported by NIH grants UO1DK072146 and RO1GM107231.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was carried out in accordance with the recommendations of the National Institutes of Health (NIH). The protocol was approved by the Institutional Review Board of the University of Pittsburgh (IRB No. 0610103).
References
- Veith NT, Histing T, Menger MD, et al. Helping prometheus: liver protection in acute hemorrhagic shock. Ann Transl Med 2017;5:206. [Crossref] [PubMed]
- Konishi T, Lentsch AB. Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration. Gene Expr 2017;17:277-87. [Crossref] [PubMed]
- Sadowsky D, Zamora R, Barclay D, et al. Machine Perfusion of Porcine Livers with Oxygen-Carrying Solution Results in Reprogramming of Dynamic Inflammation Networks. Front Pharmacol 2016;7:413. [Crossref] [PubMed]
- Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol 2001;161:Iii-xiii, 1-151.
- Kyffin JA, Sharma P, Leedale J, et al. Impact of cell types and culture methods on the functionality of in vitro liver systems - A review of cell systems for hepatotoxicity assessment. Toxicol In Vitro 2018;48:262-75. [Crossref] [PubMed]
- Ziraldo C, Vodovotz Y, Namas RA, et al. Central role for MCP-1/CCL2 in injury-induced inflammation revealed by in vitro, in silico, and clinical studies. PLoS One 2013;8:e79804. [Crossref] [PubMed]
- Vodovotz Y, Simmons RL, Gandhi CR, et al. "Thinking" vs. "Talking": Differential Autocrine Inflammatory Networks in Isolated Primary Hepatic Stellate Cells and Hepatocytes under Hypoxic Stress. Front Physiol 2017;8:1104. [Crossref] [PubMed]
- Mi Q, Constantine G, Ziraldo C, et al. A dynamic view of trauma/hemorrhage-induced inflammation in mice: principal drivers and networks. PLoS One 2011;6:e19424. [Crossref] [PubMed]
- Namas RA, Mi Q, Namas R, et al. Insights into the Role of Chemokines, Damage-Associated Molecular Patterns, and Lymphocyte-Derived Mediators from Computational Models of Trauma-Induced Inflammation. Antioxid Redox Signal 2015;23:1370-87. [Crossref] [PubMed]
- Zamora R, Korff S, Mi Q, et al. A computational analysis of dynamic, multi-organ inflammatory crosstalk induced by endotoxin in mice. PLoS Comput Biol 2018;14:e1006582. [Crossref] [PubMed]
- Metukuri MR, Beer-Stolz D, Namas RA, et al. Expression and subcellular localization of BNIP3 in hypoxic hepatocytes and liver stress. Am J Physiol Gastrointest Liver Physiol 2009;296:G499-509. [Crossref] [PubMed]
- Zamora R, Vodovotz Y, Mi Q, et al. Data-Driven Modeling for Precision Medicine in Pediatric Acute Liver Failure. Mol Med 2017;22:821-9. [Crossref] [PubMed]
- An G, Vodovotz Y. Complex Systems and Computational Biology Approaches to Acute Inflammation. Springer,2013.
- Almahmoud K, Namas RA, Zaaqoq AM, et al. Prehospital Hypotension Is Associated With Altered Inflammation Dynamics and Worse Outcomes Following Blunt Trauma in Humans. Crit Care Med 2015;43:1395-404. [Crossref] [PubMed]
- Namas RA, Vodovotz Y, Almahmoud K, et al. Temporal Patterns of Circulating Inflammation Biomarker Networks Differentiate Susceptibility to Nosocomial Infection Following Blunt Trauma in Humans. Ann Surg 2016;263:191-8. [Crossref] [PubMed]
- Galindo M, Santiago B, Alcami J, et al. Hypoxia induces expression of the chemokines monocyte chemoattractant protein-1 (MCP-1) and IL-8 in human dermal fibroblasts. Clin Exp Immunol 2001;123:36-41. [Crossref] [PubMed]
- Mawet E, Shiratori Y, Hikiba Y, et al. Cytokine-induced neutrophil chemoattractant release from hepatocytes is modulated by Kupffer cells. Hepatology 1996;23:353-8. [Crossref] [PubMed]
- Thornton AJ, Ham J, Kunkel SL. Kupffer cell-derived cytokines induce the synthesis of a leukocyte chemotactic peptide, interleukin-8, in human hepatoma and primary hepatocyte cultures. Hepatology 1991;14:1112-22. [PubMed]
- Kietzmann T. Liver Zonation in Health and Disease: Hypoxia and Hypoxia-Inducible Transcription Factors as Concert Masters. Int J Mol Sci 2019;20. [Crossref] [PubMed]
- Namas RA, Metukuri MR, Dhupar R, et al. Hypoxia-induced overexpression of BNIP3 is not dependent on hypoxia-inducible factor 1alpha in mouse hepatocytes. Shock 2011;36:196-202. [Crossref] [PubMed]
- Laurens M, Defamie V, Scozzari G, et al. Hypoxia-reoxygenation-induced chemokine transcription is not prevented by preconditioning or intermittent hypoxia, in mice hepatocytes. Transpl Int 2005;18:444-52. [Crossref] [PubMed]
- Alexopoulos LG, Saez-Rodriguez J, Cosgrove BD, et al. Networks Inferred from Biochemical Data Reveal Profound Differences in Toll-like Receptor and Inflammatory Signaling between Normal and Transformed Hepatocytes. Mol Cell Proteomics 2010;9:1849-65. [Crossref] [PubMed]
- Sullivan M, Galea P, Latif S. What is the appropriate oxygen tension for in vitro culture? Mol Hum Reprod 2006;12:653. [Crossref] [PubMed]


