
Micronutrient management following bariatric surgery: the role of the dietitian in the postoperative period
Introduction
Obesity rates in both the developing and developed world are increasing in prevalence. WHO has reported that over 10% of the world’s population (650 million people) were classified as obese in 2016, which represents a 3-fold increase in prevalence of obesity since 1975 (1). This has serious ramifications for individual health as well as national health care systems, because obesity is recognized as a major risk factor in the development of a number of chronic conditions, including cardiovascular disease, diabetes, musculoskeletal disorders and cancers such as colon, breast and prostate (1).
Treatment options for obesity have traditionally been focused on lifestyle interventions (diet and exercise) under the guidance of nutrition professionals, such as dietitians. However, in recent decades obesity treatments have trended towards medical and surgical options such as pharmacotherapy interventions, bariatric surgical procedures and more recently, endoscopic bariatric interventions. A 2014 Cochrane review of 22 randomized controlled trials described and compared surgical (bariatric surgery) versus non-surgical treatment options for obesity (diet, exercise, pharmacotherapy) found that the surgical management was more beneficial in terms of weight changes, quality of Life (QoL) and diabetes outcomes at one to two years of follow up (2). However, no relative effect size (95% confidence interval) was able to be pooled for these outcomes due to differences in participants, the type of surgery or other comparators (2).
Due to the widely reported benefits of bariatric surgery, its utilization as a treatment strategy for obesity continues to increase. In the US total number of bariatric procedures performed increased 40% between 2011 and 2017, with nearly a quarter of a million procedures being performed reported in 2017 (3). As well as increasing in incidence, there has been a shift away from the traditionally favored gastric bypass procedure (GBP) and adjustable gastric banding (AGB) towards vertical sleeve gastrectomy (SG) (3). Severely malabsorptive procedures such as biliary-pancreatic bypass, usually accompanied by duodenal switch (BPD-DS), remain relatively rarely performed (3). Additionally, there is an increase trend in the need for revisional procedures, either to mitigate complications such as the development of de novo gastro-esophageal reflux or manage weight recidivism in purely restrictive procedures (3).
Given the implications of anatomical and physiological changes following bariatric surgery, nutritional counselling and monitoring is imperative postoperatively to ensure the long-term benefits of weight loss and improvements in chronic disease management are not inadvertently giving rise to the development of nutritional sequelae. While all health care professionals treating bariatric surgery patients should be aware of these issues, dietitians are well placed to direct this aspect of postoperative follow up support (4). The role of the dietitian extends throughout the continuum of care in bariatric surgery, including the provision of pre-, and postoperative nutritional assessment and counselling, as well as foodservice support during the hospital admission (5). However, the current article will focus on the role of the dietitian within in a multidisciplinary team in longer-term micronutrient management following bariatric surgery, and the dietetic management of micronutrient (vitamin and trace element) deficiency risk following bariatric surgery.
Nutritional implications of bariatric procedures
Irrespective of the procedure utilized, bariatric surgical procedures alter the anatomy and physiology of the proximal and/or distal part of the gastrointestinal tract with a view to facilitate weight loss. All procedures involve a reduction in gastric capacity by altering the size of the stomach (restrictive effect), while the GBP and BPD-DS add a diversion of varying lengths of the proximal small bowel to reduce the absorption of food consumed (malabsorptive effect) (6). Bariatric procedures with malabsorptive components have a greater impact on long-term nutritional outcomes than restrictive procedures, and the long-term nutritional risks proportionally increases with the amount of small bowel bypassed. Table 1 describes the bariatric procedures in terms of the anatomical and hormonal changes they produce, as well as the micronutrients most commonly affected by these procedures.

Full table
Potential nutritional risks may be attributed to a number of causes. First, pre-existing micronutrient deficiencies are not uncommon findings in bariatric surgery candidates (11-13). The nutrients most commonly affected are vitamins B12 and D, folate and iron, and to date this has been attributed to poor dietary quality, with low dietary micronutrient sources relative to caloric intake (12). As preoperative screening does not yet form part of bariatric surgical management guidelines (14,15), undetected and uncorrected deficiencies at baseline may exaggerate postoperative findings.
Second, all bariatric procedures reduce the volume of food and fluids able to be consumed, thereby reducing caloric (and nutritional) intake. At the most basic level, this is facilitated by a reduced gastric capacity due to the small gastric pouch fashioned during surgery (6,16). Reduced appetite, believed to be mediated by changes in gastric and intestinal hormonal communication due to changes post-surgery, may further contribute to a reduction in the volume of nutritional intake (6). Further unintended reduction in intake may occur as a result of new or exacerbated upper gastrointestinal symptoms such as reflux or vomiting, and the subsequent development of food aversion following bariatric surgery. Furthermore, postoperative taste and olfactory changes have been observed which has been shown to impact dietary intake and food choices (16,17). The net result of these changes may lead to a nutritionally inadequate intake, especially if food variety and nutritional quality is limited (4). This has been demonstrated in a cohort of patients one year after AGB surgery, where micronutrient intake from dietary sources was shown to be significantly below the dietary recommendations for the general population (18). Ensuring a judicious, nutrient rich coverage of all food groups is required to avoid nutritional risk and diet related disease risk, and ongoing dietetic follow up and education offers a means to optimize these recommendations for individualized tolerance and food preferences.
Third, the anatomical modifications fundamental to bariatric surgery have an impact on the ability for normal digestion to occur. In restrictive procedures, surgical alteration to the stomach not only limits gastric capacity, but also impacts the ability of the stomach to churn and process chyme thoroughly (10,16). This occurs by restricting/delaying access to the pylorus (GBP, AGB) or surgically reducing its size (GBP, GS, BPD-DS). This has implications for protein digestion and access to the micronutrients that need to be released from their protein food sources to enable digestion (i.e., iron from red meat) (16). Similarly, surgical resection or bypass of the gastric cells that release hormones/enzymes key to digestive processes (i.e., intrinsic factor required for B12 absorption; stomach acid to facilitate protein digestion) also need to be considered in the postoperative follow up (16).
Dumping syndrome, an unintentional outcome of the anatomical changes associated with bariatric surgery, may further contribute to the development of postoperative nutritional problems. Early dumping, which occurs within one hour of eating, is caused by gastric emptying of hyperosmolar content into the duodenum or small bowel, with subsequent shifts in intravascular fluid into the intestinal lumen (19). It is characterized by abdominal pain, nausea, diarrhea, fatigue, palpitations and tachycardia (19). Late dumping, on the other hand, occurs one to three hours after eating carbohydrates which results from the intravascular compartment in a postprandial reactive hypoglycemia occurring in response to hyperinsulinemia (19). Symptoms can include sweating, tremors, poor concentration, altered consciousness, palpitations and syncope (19). Dumping is most commonly reported following GBP, though may occur after other procedures (19,20). Reports following GBP suggest prevalence ranging from zero to 70% in the first 6 to 24 months postoperatively (19) and in approximately 25% of patients undergoing SG (20); there is some indication that rates may vary with surgical technique utilized (19,21). While symptoms of dumping are often reported to reduce in severity over time (19,22), their presence may have significant impacts on the development of food avoidance and aversions, volume of intake tolerated and loss of nutrients through malabsorption (22). As well as affecting nutritional status, the presence or potential for dumping may confound the results of the Oral Glucose Tolerance Test (OGTT) used for the investigation of diabetes. This is particularly important to be aware of as the OGTT is the standard screening test used to diagnose gestational diabetes mellitus in pregnancy. In addition to the unreliability demonstrated by altered glucose kinetic profiles during the OGTT in pregnant women post GBP (23,24), occurrence of hypoglycemia during the OGTT (GBP 50–83%, SG 54%, AGB 12%) poses a significant risk to maternal and fetal safety, and thus should never be used in this population (25,26).
In malabsorptive procedures (GBP, BPD-DS), nutrient absorption is also impacted proportionally to the limb length, and thus the remaining alimentary limb length that reconnects chyme with pancreatic enzymes and biliary secretions (6,10,16). The length of the roux limb ultimately limits the duration of action bile and digestive enzymes can have on the food consumed, as well as the length of small bowel lumen it has access to through which to be absorbed (16). The net result yields an intentional malabsorption of consumed nutrients, which includes micronutrients.
An additional consideration in the development of micronutrient deficiencies in malabsorptive procedures may be the presence of small bowel bacterial overgrowth (SIBO). Though this is thought to be relatively rare, SIBO has been associated with the development of vitamin deficiencies such as iron, thiamine, vitamin B12 and fat-soluble vitamins due to the bacteria competing for utilization of these micronutrients with their host (27-29).
Further to anatomical and physiological changes, the presence of underlying disordered eating patterns may negatively impact on the nutrition risk experienced following bariatric surgery. Issues observed in practice range from emotional/comfort eating and issues associated with body image, to behaviors meeting eating disorder diagnostic criteria (30). Binge eating pre- and post-bariatric surgery is common finding in this patient population. A recent examination of bariatric surgery candidates identified that 16% were identified as being diagnostic of binge eating disorder and 8% as bulimia nervosa using DSM-5 criteria (31). Binge eating behavior, binge eating disorders and loss of control eating in the years following a range of bariatric surgical procedures (1 to 14 years; BPD-DS, GBP) has been associated with reduced postoperative weight loss and increased weight regain (32); however, no investigation of other nutritional outcomes beyond impact on weight loss as been undertaken to date. The unmasking and early presentation of these issues are often identified by the dietitian through the unique line of questioning undertaken during a nutritional assessment. For this reason, a multidisciplinary approach to holistic patient care, including access to both dietetic and psychological support, is important for patients to optimize their surgical outcomes while managing the potential adverse nutritional consequences of bariatric surgery.
Micronutrient management following bariatric surgery
Micronutrient deficiencies are relatively common following bariatric procedures (4), and a comprehensive review of the micronutrient vulnerabilities in this patient population have recently been described by Via and Mechanick (10). In view of the long-term nutritional considerations following primary procedure bariatric surgery, guidelines around nutritional management, prescription and monitoring have been developed and expanded over the last decade (14,15,33).
Monitoring
While there is little empirical evidence to support the timeframes, current guidelines unanimously recommend that micronutrient monitoring occur at 1, 3 to 6 and 12 months post-operatively with varying different micronutrients recommended to be tested depending on the surgery type (14,15,33). Specifically, this includes:
- Routine monitoring of iron, folate, B12, vitamin D for GBP, BPD-DS and SG (14,15,33);
- Monitoring of zinc, copper for malabsorptive procedures (GBP and BPD-DS) or in cases of otherwise unexplained or unresponsive clinical phenomena for other procedure (14,15);
- 6 to 12 monthly screening of Vitamin A in all patients in the first year (11), or following BPD and BPD-DS, and in GBP as required (14);
- Review of serum thiamine levels in cases of otherwise unexplained or unresponsive clinical phenomena in all bariatric surgery (14).
Thereafter monitoring recommendations are based on the risks posed by specific procedures and are targeted at specific nutrients (14,15). Parrott et al. recommend annual screening for folate and iron in all patients, and at-risk nutrients (vitamin B12, zinc, copper) in at-risk patients (15).
Routine supplementation
Blanket recommendations for daily multivitamin and multimineral supplementation (as a specified source of iron, folate and iron), calcium, vitamin D, vitamin B12 and iron are specifically recommended for all procedures (14,15). The Clinical Practice Guidelines 2013 update specifies that these recommendations cover the initial postoperative phase (3 to 6 months) for GBP, SG and AGB, and that supplementation should be provided in chewable forms to maximize absorption (14). The American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for Surgical Weight Loss 2016 update support and expand on these recommendations, including extending recommendations to BPD-DS procedures (see Table 2). These recommendations are considered to be the minimum ongoing requirement for patients following bariatric surgery, and that particularly in malabsorptive procedures, routine supplementation is a lifelong requirement irrespective of adequacy of oral intake.
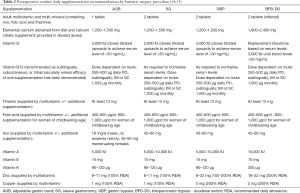
Full table
There are no specific or altered recommendations for those undergoing revisional bariatric procedures, however, this patient group will be at high nutritional risk due to their previous bariatric surgery. Preoperative assessment for and reversal of any existing nutritional deficiencies is therefore important.
Correction of deficiency
When laboratory findings indicate low serum levels of micronutrients, replacement with a view to repletion of body stores is indicated. Parrot et al. (15) provide detailed recommendations around replacement protocols following ABG, SG, GBP and BPD-DS. Treatment of suspicion or evidence of micronutrient deficiency should also be accompanied by a dietetic review to ensure dietary sources are optimized, and to identify any previously undetected causes for the deficiencies’ development.
While these recommendations and guidelines are based on the best currently available evidence, it should be noted that the course of postoperative micronutrient status is poorly described in the literature. The varied measures used and gaps in reporting on micronutrient status, replacement practices and compliance with postoperative micronutrient regimens have been highlighted in a recent systematic review that focused on GBP, SG and AGB (11). Out of the 69 articles included in the systematic review, only 22 reported on vitamin and mineral supplemental dosage intake, which is an important contributor when considering deficiency rates given the international guideline recommendations for blanket provision of two multivitamins per day (11). Similarly, a Cochrane review which reported on adverse outcomes comparing non-surgical and surgical treatments concluded that adverse outcomes, including micronutrient deficiencies, were inconsistently reported on, and were based on very low-quality evidence (2). This is an area that requires a greater degree of attention in future studies as it remains a significant gap in interpreting the existing literature, and in gaining a fuller understanding of the long-term micronutrient implications of bariatric surgery. As well as contributing to individual patient management, dietetic contributions will be important in informing future research in this area of bariatric surgical practice.
The role of the dietitian in managing micronutrient status following bariatric surgery in adults
Dietetic review for reassessment and dietary intervention in the years following bariatric surgery, along with micronutrient monitoring, supplementation and replacement as required, form an integral part to ongoing nutritional care to identify, prevent and treat micronutrient deficiencies, while continuing to support and facilitate weight loss. This is most effectively accomplished within a multidisciplinary team environment where communication of identified nutritional concerns can be acted on with a multi-pronged approach, including a targeted medical nutrition therapy intervention, supported by medical and/or psychological input where indicated. Creating a supportive and empathetic environment in which the patient feels understood and supported through the therapeutic relationship developed with their clinicians is important to facilitate the desired post-surgical outcomes.
The Nutrition Care Process undertaken by a dietitian follows a systematic process that involves assessment and interpretation of all nutritionally relevant data (clinical, nutritional, social, biochemical, behavioral), leading a nutritional diagnosis to be acted on (34). Nutritional interventions and prescriptions are negotiated between the dietitian and the patient to address the etiology of the diagnosed nutritional problem, and implementation plans and strategies are determined with a view to achieving mutually agreed goals (34). Finally monitoring and evaluation continues with periodic review, ultimately leading to reassessment and a repeating of the process to ensure the ongoing nutritional requirements of the patient are met (34).
Nutrition assessment and nutritional diagnosis
The basis of the nutrition assessment or reassessment involves taking a detailed food intake history, which incorporates an assessment of adequacy across food groups and micronutrient categories (34). Specifically, in the post bariatric surgical population this should also include assessment of:
- Global assessment of dietary patterns and food/fluid intake;
- Targeted enquiry around intake of identified vulnerable food groups/micronutrient sources specific to the bariatric procedure that has been undertaken;
- Assessment of compliance with routine micronutrient supplementation;
- Presence of GI symptoms affecting food intake such as reflux, vomiting, or other impediments to eating;
- Behavioral attitudes towards food such as food aversions, food fears, or indications of disordered body image or disordered eating patterns.
Routine laboratory values obtained through micronutrient monitoring can be interpreted in the context of the assessment, vulnerabilities identified and a diagnosis of the nutritional problem made.
Nutrition interventions
Dietary interventions
Protein foods are also rich sources of important micronutrients such as iron, calcium and B12, so the fact that protein intake is often compromised following bariatric surgery, with few patients meeting the recommended minimum of 60 g protein per day (12) has significant ramifications for micronutrient intake. Indeed, it is this lower intake of protein-providing food groups that account for the routine postoperative micronutrient supplementation recommendations. One key reason for lower postoperative protein intakes is that these foods are among most commonly associated with postoperative food aversions—one third of patients develop avoidance symptoms to common meat products and a further 12% experience similar aversions to dairy foods (17,35). A dietitian can address this finding through provision of tailored practical advice such as eating protein containing foods first, and food substitutions that provide roughly comparable protein/micronutrient provision, or alternative preparation options that may facilitate tolerance or acceptance. Table 3 summarizes nutrient sources and potential alternatives that may assist in meeting nutritional requirements.
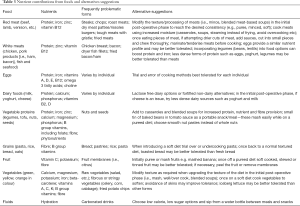
Full table
When these protein aversions or tolerance issues cannot be overcome through substitution or modification, supplementation with protein powders may be required, and have been shown to successfully meet protein requirements (36). Micronutrient fortified protein powders may be utilized where more global nutritional deficits are identified, however further supplementation may be required to compensate for intake deficits.
Dietitians are experts in the relationship between nutrients and food, and therefore dietary focused strategies are a dietitian’s first line of intervention. However, due to the anatomical and physiological changes following bariatric surgery, dietary interventions will often be used concurrently with supplementation of the affected nutrient.
Behavioral interventions
Dietary pattern and food related behaviors represent an etiological factor in nutritional vulnerabilities, including micronutrients. If this is identified to be the case, addressing these with reorienting habits towards more beneficial behaviors is required. These often represent significant changes compared to pre-surgical eating habits, and often require ongoing reinforcement by the bariatric surgical team, particularly the dietitian.
Establishing a regular eating pattern of 6 to 8 or 10 small, regular meals per day with a focus of food volume of around half a cup of high nutritional value foods are required to ensure nutrient requirements are met (4,37). This assists in avoiding missing meals, and gravitating towards larger meals which may precipitate reflux, vomiting and epigastric pain. In many cases this represents a significant change to the patient’s meal preparation and planning practices to enable appropriate food choices to be available when and where required. Dietitians may assist in bridging the gap between historical habits and acquisition of the new behaviors required post-surgery through the provision of practical advice such as recipes, meal plans, portion management and managing social situations in which food is prominent.
Avoidance of fluids at meal times is a key strategy to optimize nutritional intake through prioritizing gastric capacity for nutrients, and refocusing on hydration at other times of the day (4,37). This strategy is also helpful for postoperative symptom management such as regurgitation, upper gastric pain and reflux (4). Not having fluids available at meal times, and refocusing fluid to taking smaller volumes from a water bottle throughout the day are practical changes to the meal time environment that can assist with this aspect of nutritional management (37).
Actively slowing a meal down is also a post-surgical food-related behavior that needs to be encouraged (37). Chewing food well to release more nutrients and avoid large particles that may block the narrower gastric capacity is vital. This may be assisted further by a deliberate attention to smaller particle sizes of foods consumed (37). In some cases, environmental modification such as changing to smaller cutlery options, and placing timepieces near the meal table to time the duration of mastication and/or meal consumption may be of assistance in changing lifelong pre-surgical habits (37).
Finally, the concept of mindful eating—bringing a conscious awareness to the food being consumed, including the taste, mouth-feel, appearance, temperature, etc. is a valuable tool for managing intake (37). Dietitians may promote practical strategies such as avoiding food when doing other tasks (such as watching TV or working at the computer) as well as introduce basic techniques to promote mindful eating. These foundational principles may be further expanded with input from the psychologist within the multidisciplinary team for more specific training in these skills.
To achieve the identified long-term nutritional goals, a dietitian may be required to take on aspects of the role of ‘life coach’ for patients following bariatric surgery, while drawing on their expert nutrition knowledge. This may occur where there remains a deficit between the patient’s knowledge of what is required and their skills or confidence to implement the required changes. Examples may include the dietitian assisting in patients acquiring food preparation skills, providing an avenue for accountability with changes made, and troubleshooting situations such as eating out, social events and holiday seasons.
Monitoring and evaluation
Variance from the anticipated outcomes in micronutrient management following bariatric surgery will likely require escalation to intensive pharmaceutical, medical and/or surgical interventions to effectively manage the underlying situation. Similarly, follow up intervals for dietetic review are determined through monitoring and evaluation phases (34).
These specific considerations around the Nutrition Care Process may be further adapted and tailored to address nutritionally significant situations in postoperative dietetic management of bariatric surgery, such as supporting women with a history of bariatric surgery through preconception, pregnancy and lactation.
Pregnancy and lactation following bariatric surgery, and the role of the dietitian
Pregnancy and lactation are times of increased nutritional demand. Maternal micronutrient requirements increase by 10–50% during pregnancy to accommodate metabolic and hemodynamic changes, rapid cell division, and fetal development (38). Requirements substantially increase for many micronutrients during pregnancy such as iodine, folate, iron and zinc, and remain elevated to support lactation (38). As many of these micronutrients are already recognized as vulnerabilities following bariatric surgery, women with a history of bariatric surgery are at greater micronutrient risk during these stages of the lifecycle. In addition to the limitations of their post-surgical anatomy, physiology, and food behaviors, during pregnancy nutritional deficiencies may be further exacerbated by common pregnancy symptoms (39).
Table 4 outlines the micronutrient requirements pre-conception, during pregnancy and lactation, as well as guidance around supplementation during pregnancy for women with a history of bariatric surgery. Currently no international guidelines provide micronutrient recommendations specifically for pregnancy and lactation post-bariatric surgery. Suggested supplementation is based on expert opinion and modification of professional body guidelines for non-pregnant patients (15,38-42). This is still a developing area owing to the relatively recent increase in rates of bariatric surgery performed in women of childbearing age (25). Accordingly, procedure specific micronutrient recommendations or supplementation advice does not exist; although as with the non-pregnant population, deficiency risk may be higher in more malabsorptive procedures (25). However, given how critical this period is for fetal development and the potential implications of deficiency, frequent micronutrient surveillance is suggested irrespective of surgery type (40).
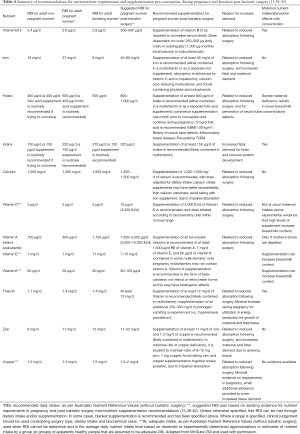
Full table
A 2019 systematic review of 27 studies (25) reported micronutrient deficiencies in pregnant women post-bariatric surgery were more prevalent for iron, vitamin B12, vitamin D, vitamin A, zinc, and vitamin K; less frequently cited were deficiencies in vitamin E, B1, B6, C, folate and selenium. Adverse maternal outcomes associated with these have been reported to include to anemia (vitamin B12, iron), night blindness (vitamin A), and urinary tract infections (vitamins A and D) (25). Adverse neonatal outcomes related to maternal micronutrient deficiencies have been reported to include visual complications (vitamin A), intracranial hemorrhage (vitamin K), neurological and developmental impairment (vitamin B12), and neural tube defects (folate) (43,44). In terms of lactation, there is little evidence reporting clinically significant changes in breastmilk composition in women post-bariatric surgery when compared to controls (45-48) and deficiencies in exclusively breastfed infants is restricted to case reports of vitamin B12 deficiency (49-51). Given that the breastfeeding infant relies on breastmilk to supply the majority of micronutrients (excluding vitamin B6 and vitamin K), the risk of fetal and maternal deficiency remains a concern (40). However, there are many methodological issues with the currently available evidence, as many studies have poor quality study designs, do not report on supplementation protocol or adherence, dietary intake, micronutrient serum levels, deficiency criteria, surgery type, time to conception, and often have no control group (25,43,47,48,52). Investigation of the rates of maternal deficiency during lactation after bariatric surgery is also limited and existing papers have conflicting results and very short follow up (47,52). Breastfeeding practices are also poorly described in the literature, for example, Gimenes and colleagues studied micronutrient levels of post-natal women after bariatric surgery but did not report on breastfeeding behaviors (52).
Given the potential impact of micronutrient deficiencies in this population, along with evidence suggesting the nutrition status of the mother is a critical factor in ‘fetal programming’ and the child’s long-term chronic health risks (39), dietetic involvement in the multidisciplinary care of women of childbearing age following bariatric surgery is imperative.
Dietitians should be actively involved with pre-conception nutrition counselling of planned pregnancies and as early as feasible in unplanned pregnancies after bariatric surgery. Guidelines currently recommend delaying pregnancy 12 to 24 months after bariatric surgery (14,53), due to induced catabolic state and rapid weight-loss (54). This aims to reduce the risk of intrauterine growth restriction, whilst allowing women to maximize weight-loss and metabolic outcomes resulting from bariatric surgery (54). The role of the dietitian pre-conception should include but not be limited to: identification and correction of pre-existing micronutrient deficiencies—especially folate; optimizing management of postsurgical symptoms, food aversions or food related behaviors; adherence to routine postoperative micronutrient supplementation; and consideration of dietary quality with a view to meet pregnancy nutritional requirements.
The role of a dietitian during pregnancy includes the management of pregnancy-related symptoms which further exacerbate the risk of micronutrient deficiencies in this population (39). These symptoms include morning sickness/hyperemesis (nausea, vomiting, anorexia), food aversion related to increased olfactory sensitivity and dysgeusia, gastro-esophageal reflux, constipation, and increasing abdominal pressure (39). Individualized advice by a dietitian experienced in both bariatric surgery and maternal nutrition may assist in navigating these symptoms to prevent adverse nutritional, pregnancy or offspring outcomes. Dietary interventions do not vary from those outlined in Table 3, however, the measurement of success will be determined by indications of a normally progressing pregnancy: maternal and fetal growth as determined by pregnancy weight gain (in context of gestation and pre-pregnancy BMI), fundal height and ultrasound scans (42). If the woman is obese, an ultrasound assessment of fetal anatomy is less accurate, however provides a superior assessment of fetal growth than other clinical measures, and thus additional ultrasound scans are advised (42).
In the post-partum setting dietitians provide individualized interventions and monitoring to both mothers and infants, beginning with the promotion and support of breastfeeding. Rates of exclusive breastfeeding for the recommended 6 months are below one third in many developed countries (55-57) with studies reporting even lower rates amongst women following bariatric surgery (52,58). Other key roles of dietitians in this life stage include advocating for micronutrient monitoring, monitoring for signs of deficiency in mother and child, advising women on practical methods to meet their increased requirements, return to pre-pregnancy weight, optimizing weight and nutritional status between pregnancies and provide adequate nutrition to their infant (through either breast or formula feeding). As the child grows, they may also play a role in fostering a healthy relationship with food and role modelling of the mother. The involvement of dietitians in research in this population also offers advantages in ensuring reliable reporting and interpretation of factors such as infant feeding practices, oral intake, supplementation and nutrition status.
Micronutrient monitoring throughout preconception, pregnancy and post-partum should be overseen by a multidisciplinary team, including an obstetrician, bariatric surgeon, midwife and dietitian (preferably with maternity or bariatric surgery experience) (38). A full micronutrient screening test to identify any deficiencies prior to conception, supplementation and dietary advice should be provided to meet recommended requirements (39). Ongoing nutritional surveillance and micronutrient screening for deficiencies should be done every trimester, and continue at a similar frequency for lactating mothers postnatally (40) (Table 5).
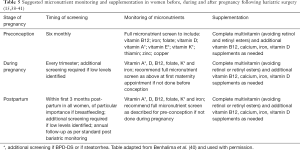
Full table
Conclusions
Bariatric surgery, though an effective method of facilitating weight loss in the obese patient, requires ongoing multidisciplinary postoperative follow up due to the subsequent risk of nutritional deficiencies. Dietitians play an important role in minimizing risk of harm to the patient in the long-term in general, as well as in specific stages of the lifecycle such conception, gestation and lactation following bariatric surgery. The role of nutrition monitoring and supplementation is addressed in a number of guidelines and remains an area of ongoing research, however there remain gaps in the literature and flaws in the methodology underpinning the research on which the international guidelines are based. Further micronutrient research post bariatric surgery is required, and dietitians have important contributions to make in this area, as well as clinical practice.
Acknowledgments
The authors wish to thank Dr Susan de Jersey for review of the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Muhammed Ashraf Memon) for the focused issue “Bariatric Surgery” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The focused issue “Bariatric Surgery” was commissioned by the editorial office without any funding or sponsorship. MAM served as the unpaid Guest Editor of the focused issue. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organsiation. Obesity and overweight 2018 [Cited 11 April 19]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014.CD003641. [PubMed]
- American Society for Metabolic and Bariatric Surgery. Estimate of Bariatric Surgery Numbers, 2011-2017 2018 [updated June 2018]. [Cited 11 April 19]. Available online: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers
- Sherf Dagan S, Goldenshluger A, Globus I, et al. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Adv Nutr 2017;8:382-94. [Crossref] [PubMed]
- Kulick D, Hark L, Deen D. The bariatric surgery patient: a growing role for registered dietitians. J Am Diet Assoc 2010;110:593-9. [Crossref] [PubMed]
- Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology 2007;132:2253-71. [Crossref] [PubMed]
- Mulla CM, Middelbeek RJW, Patti M-E. Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann N Y Acad Sci 2018;1411:53-64. [Crossref] [PubMed]
- Kotidis EV, Koliakos G, Papavramidis TS, et al. The effect of biliopancreatic diversion with pylorus-preserving sleeve gastrectomy and duodenal switch on fasting serum ghrelin, leptin and adiponectin levels: is there a hormonal contribution to the weight-reducing effect of this procedure? Obes Surg 2006;16:554-9. [Crossref] [PubMed]
- Stratis C, Alexandrides T, Vagenas K, et al. Ghrelin and peptide YY levels after a variant of biliopancreatic diversion with Roux-en-Y gastric bypass versus after colectomy: a prospective comparative study. Obes Surg 2006;16:752-8. [Crossref] [PubMed]
- Via MA, Mechanick JI. Nutritional and Micronutrient Care of Bariatric Surgery Patients: Current Evidence Update. Curr Obes Rep 2017;6:286-96. [Crossref] [PubMed]
- Lewis CA, de Jersey S, Hopkins G, Hickman I, Osland E. Does Bariatric Surgery Cause Vitamin A, B1, C or E Deficiency? A Systematic Review. Obes Surg 2018;28:3640-57. [Crossref] [PubMed]
- Roust LR, DiBaise JK. Nutrient deficiencies prior to bariatric surgery. Curr Opin Clin Nutr Metab Care 2017;20:138-44. [Crossref] [PubMed]
- Caron M, Hould FS, Lescelleur O, et al. Long-term nutritional impact of sleeve gastrectomy. Surg Obes Relat Dis 2017;13:1664-73. [Crossref] [PubMed]
- Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient-2013 update: Cosponsored by American association of clinical endocrinologists, the obesity society, and American society for metabolic & bariatric surgery. Obesity 2013;21:S1-27. [Crossref] [PubMed]
- Parrott J, Frank L, Rabena R, et al. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg Obes Relat Dis 2017;13:727-41. [Crossref] [PubMed]
- Steenackers N, Gesquiere I, Matthys C. The relevance of dietary protein after bariatric surgery: what do we know? Curr Opin Clin Nutr Metab Care 2018;21:58-63. [Crossref] [PubMed]
- Zerrweck C, Zurita L, Alvarez G, et al. Taste and Olfactory Changes Following Laparoscopic Gastric Bypass and Sleeve Gastrectomy. Obes Surg 2016;26:1296-302. [Crossref] [PubMed]
- McGrice MA, Porter JA. The micronutrient intake profile of a multicentre cohort of Australian LAGB patients. Obes Surg 2014;24:400-4. [Crossref] [PubMed]
- Ramadan M, Loureiro M, Laughlan K, et al. Risk of Dumping Syndrome after Sleeve Gastrectomy and Roux-en-Y Gastric Bypass: Early Results of a Multicentre Prospective Study. Gastroenterol Res Pract 2016;2016:2570237.
- Ahmad A, Kornrich DB, Krasner H, et al. Prevalence of Dumping Syndrome After Laparoscopic Sleeve Gastrectomy and Comparison with Laparoscopic Roux-en-Y Gastric Bypass. Obes Surg 2019;29:1506-13. [Crossref] [PubMed]
- Mallory GN, Macgregor AM, Rand CS. The Influence of Dumping on Weight Loss After Gastric Restrictive Surgery for Morbid Obesity. Obes Surg 1996;6:474-8. [Crossref] [PubMed]
- Ukleja A. Nutritional Issues in Gastroenterology, Series #35 Dumping Syndrome. Practical Gastroenterology 2006.32-46.
- Feichtinger M, Stopp T, Hofmann S, et al. Altered glucose profiles and risk for hypoglycaemia during oral glucose tolerance testing in pregnancies after gastric bypass surgery. Diabetologia 2017;60:153-7. [Crossref] [PubMed]
- Göbl CS, Bozkurt L, Tura A, et al. Assessment of glucose regulation in pregnancy after gastric bypass surgery. Diabetologia 2017;60:2504-13. [Crossref] [PubMed]
- Rottenstreich A, Elazary R, Goldenshluger A, et al. Maternal nutritional status and related pregnancy outcomes following bariatric surgery: A systematic review. Surg Obes Relat Dis 2019;15:324-32. [Crossref] [PubMed]
- Freitas C, Araujo C, Caldas R, et al. Effect of new criteria on the diagnosis of gestational diabetes in women submitted to gastric bypass. Surg Obes Relat Dis 2014;10:1041-6. [Crossref] [PubMed]
- Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y) 2007;3:112-22. [PubMed]
- Decker GA, Swain JM, Crowell MD, et al. Gastrointestinal and nutritional complications after bariatric surgery. Am J Gastroenterol 2007;102:2571-80. [Crossref] [PubMed]
- Chan WW, Thompson CC, Lautz DB, et al. Tu1674 - Risk of Small Intestinal Bacterial Overgrowth in Roux-en-Y Gastric Bypass. Gastroenterology 2011;140:S-1057. [Crossref]
- Rusch MD, Andris D. Maladaptive eating patterns after weight-loss surgery. Nutr Clin Pract 2007;22:41-9. [Crossref] [PubMed]
- Williams GA, Hawkins MAW, Duncan J, et al. Maladaptive eating behavior assessment among bariatric surgery candidates: Evaluation of the Eating Disorder Diagnostic Scale. Surg Obes Relat Dis 2017;13:1183-8. [Crossref] [PubMed]
- Meany G, Conceicao E, Mitchell JE. Binge eating, binge eating disorder and loss of control eating: effects on weight outcomes after bariatric surgery. Eur Eat Disord Rev 2014;22:87-91. [Crossref] [PubMed]
- Allied Health Sciences Section Ad Hoc Nutrition Committee, Aills L, Blankenship J, et al. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis 2008;4:S73-108. [Crossref] [PubMed]
- Academy of Nutrition and Dietetics. Nutrition Terminology Reference Manual (eNCPT): Dietetics Language for Nutrition Care: Academy of Nutrition and Dietetics; 2018 [cited 2019 25/3/19]. Available online: http://www.ncpro.org
- Graham L, Murty G, Bowrey DJ. Taste, smell and appetite change after Roux-en-Y gastric bypass surgery. Obes Surg 2014;24:1463-8. [Crossref] [PubMed]
- Schollenberger AE, Karschin J, Meile T, et al. Impact of protein supplementation after bariatric surgery: A randomized controlled double-blind pilot study. Nutrition 2016;32:186-92. [Crossref] [PubMed]
- Shannon C, Gervasoni A, Williams T. The bariatric surgery patient--nutrition considerations. Aust Fam Physician 2013;42:547-52. [PubMed]
- National Health and Medical Research Council. New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand (Version 1.2). In: Australian Government Department of Health and Ageing, New Zealand Ministry of Health, editors. Canberra: National Health and Medical Research Council; 2017.
- Slater C, Morris L, Ellison J, et al. Nutrition in Pregnancy Following Bariatric Surgery. Nutrients 2017;9. [Crossref] [PubMed]
- Benhalima K, Minschart C, Ceulemans D, et al. Screening and Management of Gestational Diabetes Mellitus after Bariatric Surgery. Nutrients 2018;10. [Crossref] [PubMed]
- McGuire E. Nutritional consequences of bariatric surgery for pregnancy and breastfeeding Breastfeeding Review 2018;26:19-26. [online].
- Gynaecologists RAaNZCoOa. Management of Obesity in Pregnancy 2017 [updated March 2017]. [Cited 11 April 19]. Available online: https://www.ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women's%20Health/Statement%20and%20guidelines/Clinical-Obstetrics/Management-of-obesity-%28C-Obs-49%29-Review-March-2017.pdf?ext=.pdf
- Jans G, Guelinckx I, Voets W, et al. Vitamin K1 monitoring in pregnancies after bariatric surgery: a prospective cohort study. Surg Obes Relat Dis 2014;10:885-90. [Crossref] [PubMed]
- Jans G, Matthys C, Bogaerts A, et al. Maternal micronutrient deficiencies and related adverse neonatal outcomes after bariatric surgery: a systematic review. Adv Nutr 2015;6:420-9. [Crossref] [PubMed]
- Jans G, Devlieger R, De Preter V, et al. Bariatric Surgery Does Not Appear to Affect Women's Breast-Milk Composition. J Nutr 2018;148:1096-102. [PubMed]
- Jans G, Matthys C, Lannoo M, et al. Breast milk macronutrient composition after bariatric surgery. Obes Surg 2015;25:938-41. [Crossref] [PubMed]
- Garretto D, Kim YK, Quadro L, et al. Vitamin A and beta-carotene in pregnant and breastfeeding post-bariatric women in an urban population. J Perinat Med 2019;47:183-9. [Crossref] [PubMed]
- Persad MD, Perseleni T, Baker D, et al. Are Vitamin D Levels Lower in the Breast Milk of Bariatric Surgery Patients? Obstetrics & Gynecology 2018;131:156S-7S. [18N]. [Crossref]
- Celiker MY, Chawla A. Congenital B12 deficiency following maternal gastric bypass. J Perinatol 2009;29:640-2. [Crossref] [PubMed]
- Grange DK, Finlay JL. Nutritional vitamin B12 deficiency in a breastfed infant following maternal gastric bypass. Pediatr Hematol Oncol 1994;11:311-8. [Crossref] [PubMed]
- Wardinsky TD, Montes RG, Friederich RL, et al. Vitamin B12 deficiency associated with low breast-milk vitamin B12 concentration in an infant following maternal gastric bypass surgery. Arch Pediatr Adolesc Med 1995;149:1281-4. [Crossref] [PubMed]
- Gimenes JC, Nicoletti CF, de Souza Pinhel MA, et al. Pregnancy After Roux en Y Gastric Bypass: Nutritional and Biochemical Aspects. Obes Surg 2017;27:1815-21. [Crossref] [PubMed]
- Willis K, Lieberman N, Sheiner E. Pregnancy and neonatal outcome after bariatric surgery. Best Pract Res Clin Obstet Gynaecol 2015;29:133-44. [Crossref] [PubMed]
- Narayanan RP, Syed AA. Pregnancy Following Bariatric Surgery-Medical Complications and Management. Obes Surg 2016;26:2523-9. [Crossref] [PubMed]
- Australian Institute of Health and Welfare. 2010 Australian National Infant Feeding Survey: indicator results. Canberra: AIHW; 2011.
- Health and Social Care Information Centre IR. Infant feeding survey 2010. In: Service NH, editor. 2012. Available online: https://sp.ukdataservice.ac.uk/doc/7281/mrdoc/pdf/7281_ifs-uk-2010_report.pdf
- United Nations International Children’s Education Fund. Infant and young child feeding 2018 [Cited 29 March 19]. Available online: https://data.unicef.org/topic/nutrition/infant-and-young-child-feeding/
- Caplinger P, Cooney AT, Bledsoe C, et al. Breastfeeding outcomes following bariatric surgery. Clinical Lactation 2015;4:9.


