
Breakthroughs and challenges in the management of tropomyosin receptor kinase fusion-positive tumors
The past decade has witnessed a paradigm shift in the treatment of cancer, moving from ‘one size fits all’ to a more precision medicine-based approach owing to the development of molecular diagnostic technologies capable of identifying an increasing number of actionable genomic alterations in the tumor tissue and circulating tumor DNA. Tyrosine kinase inhibitors (TKIs) targeting epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) gene rearrangement in advanced non-small cell lung cancer are notable examples of successful molecularly targeted therapies in oncology (1,2). These therapies have not only proven to improve overall survival compared to standard chemotherapy, but are also remarkably well tolerated leading to an enormous impact on maintaining a good quality of life for patients with advanced or metastatic cancer. Historically, the role of oncogene rearrangements in tumorigenesis was considered negligible because of its very low prevalence compared with DNA sequencing alterations or amplifications. However, the discovery of therapeutic agents targeting gene rearrangements involving ALK, ROS1, and RET have now established gene fusion as one of the important therapeutic targets (3). One such gene fusion that has gained an increasing amount of interest in recent years is neurotrophic receptor tyrosine kinase (NTRK).
NTRK1, NTRK2, and NTRK3 genes encode for three transmembrane proteins, TRK A, B, and C receptors respectively, that belong to the tropomyosin receptor kinase (TRK) family. These receptor tyrosine kinases are normally expressed in human neuronal tissue, and are essential for the development and function of the nervous system (4,5). All three TRK receptors consist of an extracellular domain, a transmembrane domain, and an intracellular domain with a kinase function. The binding of the ligand to the extracellular domain triggers the oligomerization of the receptors and phosphorylation of tyrosine residues in the intracellular kinase domain. This event results in the activation of signal transduction pathways leading to proliferation, differentiation, and survival in normal and neoplastic cells. The upregulation of TRK receptors has been reported in several central nervous system-related disorders including epilepsy and depression (4,6). The fusion events involving the 3’ region of the NTRK gene and 5’ region of various partner genes by an intrachromosomal or interchromosomal rearrangement lead to overexpression of the chimeric protein, resulting in constitutively active, ligand-independent downstream signaling (2,4). These fusions lead to oncogene addiction and have been implicated in up to 1% of all solid tumors (7). Since the first report of identification of NTRK1 gene fusion in colon cancer in 1986, there have been a number of studies reporting the presence of NTRK family gene fusions in other tumor types and the signaling pathways associated with it (3,8). However, the clinical utility of this genomic alteration in the treatment of patients was largely unknown until recently.
The first major clinical breakthrough in the treatment of NTRK fusion harboring tumors was reported in early 2018. The article published by Drilon et al. in the New England Journal of Medicine reported the integrated safety and efficacy analysis of the first age- and tumor-agnostic NTRK directed therapy in three clinical studies—a phase 1 study involving adults, a phase 1–2 study involving children, and a phase 2 basket trial involving adolescents and adults (2). The eligible patients had locally advanced or metastatic non-central nervous system tumors harboring TRK fusion determined by next-generation sequencing or fluorescent in situ hybridization (FISH), had disease progression on available standard therapies, had an ECOG performance status of 0–3, and had not received prior anti-TRK therapy except for one patient who was enrolled prior to this eligibility criterion was added to the protocol. The patients received larotrectinib, a potent and highly selective oral small molecule inhibitor of all three TRK proteins, until disease progression or development of unacceptable side effects. The primary endpoint was overall response rate (ORR) assessed by independent radiology review committee utilizing RECIST 1.1 criteria, and the secondary endpoints included ORR according to investigator’s assessment, duration of response (DoR), progression free survival (PFS), and safety. The reported analysis included 55 patients, ranging from 4 months to 76 years of age, with 17 different tumor types. The most common tumor types were salivary gland tumors (22%), soft tissue sarcoma (20%), infantile fibrosarcoma (13%), and thyroid cancer (9%). The most common gene fusion was NTRK3 (53%) followed by NTRK1 (45%) and NTRK2 (2%).
At the data cut-off time, ORR was 75% (95% CI, 61–85%) according to the independent radiology review, including 13% complete responses, 62% partial responses, and 13% stable disease. The ORR was 80% (95% CI, 67–90%) per investigator’s assessment. The example of remarkable responses included two pediatric patients with locally advanced infantile fibrosarcoma who were eventually able to undergo limb sparing surgery with a curative intent and remained disease free without larotrectinib after 4.8 and 6 months of follow-up. In addition to a high response rate, larotrectinib resulted in durable responses with median DoR not reached at the median follow-up time of 8.3 months (range, 0.03–24.9 months). Response duration was 6 months or longer for 73%, 9 months or longer for 63%, and 12 months or longer for 39% of patients including a patient who had been receiving the treatment for 27 months at the time of data cut-off. Similarly, median PFS was not reached at the median follow-up time of 9.9 months (range, 0.7–25.9 months). The responses were independent of the tumor-type, patient age, and the type of NTRK gene fusion. The majority of the treatment related adverse events (TRAEs) were grade 1 or 2. Twelve patients experienced grade 3 TRAEs, including anemia, increase in AST/ALT, weight gain, and neutropenia. No grade 4 or 5 TRAEs were observed. While eight of the total 55 patients required dose reduction because of TRAEs, none of the patients discontinued treatment because of the side effects.
Based on these data, the US FDA approved larotrectinib in November 2018 for treatment of tumors harboring NTRK gene fusion that are either metastatic or where surgical resection is likely to result in severe morbidity, and who have progressed following standard treatment. This is the second tumor-agnostic FDA approval for the treatment of cancer, following approval of PD-1 inhibitor pembrolizumab for tumors with microsatellite instability, regardless of the tumor histology.
Despite the impressive results of larotrectinib in this molecularly selected subset of patients, resistance to larotrectinib, either primary or acquired, remains an invariable challenge. Given the small number of patients with primary resistance to larotrectinib on this study, the underlying mechanisms remain largely unknown except for the possibility of either a false positive result while testing for NTRK fusion, or the lack of protein-level expression of the molecularly identified fusion. The mechanism of acquired resistance is somewhat better understood with kinase domain mutations comprising of substitutions in the solvent front position (NTRK1 G959R, NTRK3 G623R), the gatekeeper position (NTRK1 F589L), and the xDFG position (NTRK1 G667S, NTRK3 G696A). These resistance mutations lead to structural changes in the kinase domain that interfere with the binding of the drug. A few patients had more than one acquired resistance mutations. Another first-generation NTRK inhibitor, entrectinib (RXDX-101), has also been reported to have a remarkable efficacy in tumors harboring TRK fusions as well as ROS1 and ALK rearrangements, with the suggestion of clinically meaningful intracranial activity and similar side effect profile as larotrectinib (9). However, development of resistance mutations in the kinase domain leading to resistance to entrectinib was again an inevitable occurrence. The emergence of resistance mutations is analogous to the phenomenon observed in other molecularly targeted therapies utilizing TKIs.
While the beginning of the story of NTRK directed therapy may seem very similar to other targeted therapies that are already approved, the most striking and rather distinctive attribute of this therapy is it’s tumor agnostic efficacy, including in rare adult and pediatric tumors that are resistant to chemotherapy such as thyroid cancer, GIST, infantile fibrosarcoma, and melanoma. Additionally, the responses are remarkable as well as durable. The advent of the therapy targeting NTRK fusion is of paramount importance because of the limited therapeutic options available for the relatively rare tumor types that are known to be enriched for TRK fusions. Nevertheless, larotrectinib and entrectinib certainly represent the first step in a uniquely long journey. Presence of primary resistance and development of acquired resistance are among the major challenges. Longer follow-up of these patients is unquestionably necessary to assess the patterns of disease progression and various resistance mechanisms other than the identified mutations. Another unanswered question is the efficacy of larotrectinib in treating brain metastasis and leptomeningeal disease considering the fact that there was only one patient with brain metastasis included on the study. It is also imperative to have a longer follow up of the patients, especially the pediatric patients, to assess whether the therapy leads to any developmental and/or neurologic impairment since TRK plays an essential role in the development and function of human neuronal tissue. To overcome some of the challenges associated with the first generation NTRK inhibitors, several second generation NTRK inhibitors with activity in tumors harboring resistance mutations are being explored in various stages of clinical development. The most notable ones are LOXO-195 and TPX-0005 (repotrectinib). As more data become available on the safety and efficacy profile of the newer NTRK inhibitors, the sequencing of these agents and its impact on overall survival of the patients will be an area of growing interest. Table 1 summarizes the completed and ongoing clinical trials utilizing various NTRK inhibitors.
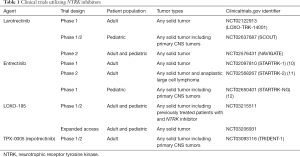
Full table
In conclusion, the advent of tumor- and age-agnostic therapy for TRK fusion harboring tumors, a number of which are rare tumor types with limited treatment options and dismal prognosis, has expanded the horizons of precision medicine-driven cancer treatment. While this newer target has brought about a monumental change in the treatment of the select subset of patients, there remain a number of unanswered questions ahead of us including the best strategy to prevent and overcome the emergence of resistance mutations, sequencing of various NTRK inhibitors, and the development and implementation of cost-effective and time-efficient tests to identify NTRK fusions. Finally, this data further supports the importance of genomic analysis in patients with advanced solid organ malignancies.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. [Crossref] [PubMed]
- Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov 2015;5:25-34. [Crossref] [PubMed]
- Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 2016;1:e000023. [Crossref] [PubMed]
- Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett 2001;169:107-14. [Crossref] [PubMed]
- Boulle F, Kenis G, Cazorla M, et al. TrkB inhibition as a therapeutic target for CNS-related disorders. Prog Neurobiol 2012;98:197-206. [Crossref] [PubMed]
- Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun 2014;5:4846. [Crossref] [PubMed]
- Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 1986;319:743-8. [Crossref] [PubMed]
- Liu D, Offin M, Harnicar S, et al. Entrectinib: an orally available, selective tyrosine kinase inhibitor for the treatment of NTRK, ROS1, and ALK fusion-positive solid tumors. Ther Clin Risk Manag 2018;14:1247-52. [Crossref] [PubMed]
- Drilon A, Siena S, Ou SI, et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov 2017;7:400-9. [Crossref] [PubMed]
- Drilon A, Sankhala KK, Liu SV, et al. Abstract CT060: STARTRK-2: A global phase 2, open-label, basket study of entrectinib in patients with locally advanced or metastatic solid tumors harboring TRK, ROS1, or ALK gene fusions. Cancer Research 2017;77:CT060. -CT.
- Desai AV, Brodeur GM, Foster J, et al. Abstract CT030: STARTRK-NG: A phase 1/1b study of entrectinib in children and adolescents with advanced solid tumors and primary CNS tumors, with or without TRK, ROS1, or ALK fusions. Cancer Research 2017;77:CT030. -CT.


