
Cardiovascular and renal protection with sodium-glucose cotransporter type 2 inhibitors: new paradigm in type 2 diabetes management…and potentially beyond
Sodium-glucose cotransporter type 2 inhibitors (SGLT2is) are new glucose-lowering agents that enhance glucosuria independently of insulin (1). They improve glucose control in patients with type 2 diabetes mellitus (T2DM) by reducing both fasting and postprandial hyperglycemia, with a minimal risk of hypoglycemia. They also promote weight loss because of the recurrent daily calorie loss in the urine. Furthermore, they induce osmotic diuresis and natriuresis, an effect that results in a reduction in systolic blood pressure and fluid overload (2). Of note, recent studies showed that SGLT2is can also reduce low-grade inflammation (3), a condition associated with atherosclerotic cardiovascular disease (ASCVD). Thus, SGLT2is improve several cardiovascular factors, beyond glucose control. However, besides all these favourable effects, SGLT2is have been shown to be associated with a variety of adverse events (4). Some may be attributed to the specific mechanism of action, such as urinary (less events than initially frightened indeed) and more common genital (mycotic) infections. Others were less expected events such as episodes of so-called euglycemic ketoacidosis, but may be explained in some predisposing circumstances. Finally, others were completely unexpected such as lower-limb (mainly toe) amputations. Whether these latter complications should be considered as a class effect (indeed most reports concerned canagliflozin) and which underlying mechanisms might be considered as causal factors remain a matter of discussion (4). Thus, the benefit-risk balance of SGLT2is should be taken into account when prescribing these glucose-lowering agents in clinical practice for the management of T2DM (5,6).
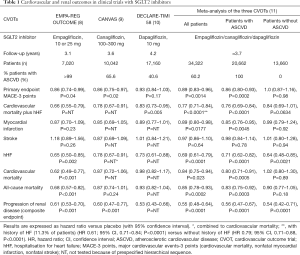
Of particular interest from a clinical point of view, SGLT2is demonstrated cardiovascular and renal protection in T2DM patients with ASCVD in three large prospective placebo-controlled cardiovascular outcome trials (CVOTs) primarily designed to prove the safety of these new glucose-lowering agents (7): EMPA-REG OUTCOME with empagliflozin (8), CANVAS program with canagliflozin (9) and DECLARE-TIMI 58 with dapagliflozin (10). Results were mainly consistent across the three trials, with a reduction in the composite primary endpoint [three-point major cardiovascular events or MACEs, i.e., cardiovascular mortality, nonfatal myocardial infarction and nonfatal stroke: significant in EMPA-REG OUTCOME and CANVAS, but not in DECLARE-TIMI 58; the latter showed a significant reduction in another prespecified primary composite outcome that combines hospitalisation for HF and cardiovascular mortality]; a reduction in cardiovascular and all-cause mortality (only significant in EMPA-REG OUTCOME); a reduction in hospitalization for heart failure (HF) (significant in all three trials); and a reduction in composite renal outcomes (significant in EMPA-REG OUTCOME and CANVAS, similar reduction in DECLARE-TIMI 58 but exploratory analysis only) (Table 1). The differences that were noticed were essentially attributed to differences in the clinical characteristics of the recruited populations. Indeed, whereas EMPA-REG OUTCOME recruited only patients with established ASCVD (a large majority with antecedents of clinical events), CANVAS selected only two-thirds of patients with established ASCVD and DECLARE-TIMI 58 only about 40% (Table 1). Another potential difference concerned the renal function, which was more deteriorated in EMPA-REG OUTCOME and CANVAS populations than in patients of DECLARE-TIMI 58. Overall, patients from EMPA-REG OUTCOME were at higher risk than patients from CANVAS and even more than patients from DECLARE-TIMI 58, a difference that resulted in a higher incidence rate of MACEs in the placebo control group.
Full table
The main results of these three CVOTs were summarized in a meta-analysis by Zelniker and colleagues, which was published in the Lancet (11) simultaneously with the report by the same group of the results from DECLARE-TIMI 58 in the New England Journal of Medicine (10) (Table 1). This meta-analysis took profit from a large pooled population—data from 34,322 patients (60.2% with established ASCVD), with 3,342 MACEs, 2,028 cardiovascular deaths or hospitalisations for HF events, and 766 renal composite outcomes—to analyse the effects of SGLT2is according to the presence or not of ASCVD, HF or chronic kidney disease (CKD) at baseline. SGLT2is reduced MACEs events by 11%, with benefit only seen in patients with ASCVD and not in those without (P for interaction =0.0501) (Table 1). Compared with placebo, SGLT2is reduced the risk of cardiovascular death or hospitalisation for HF by 23%. Of note, the benefit was almost similar in patients with and without ASCVD (P for interaction =0.41) (Table 1) and with and without a history of HF (P for interaction =0.51). SGLT2is also reduced the risk of progression of renal disease by 45%, again with a similar benefit in patients with and without ASCVD (P for interaction =0.71) (Table 1). All three trials recruited patients with a rather large range of estimated glomerular filtration rate (eGFR) so that it was possible to compare the results in patients with eGFR <60 versus >60 mL/min/1.73 m2. Interestingly, the magnitude of benefit of SGLT2is varied with baseline renal function. Indeed, greater reductions in hospitalisations for HF (P for interaction =0.0073, but lower reductions in progression of renal disease (P for interaction =0.0258) were observed in patients with more severe CKD at baseline (11). The conclusion of the authors was that SGLT2is have moderate benefits on atherosclerotic MACEs and that these positive effects seem confined to patients with established ASCVD. However, the beneficial effects in reducing hospitalisation for HF and progression of renal disease appear robust regardless of existing ASCVD or a history of HF. These conclusions were shared and reinforced in an editorial in the same issue of the Lancet by Verma and colleagues (12). These authors proposed a schematic illustration of main protective effects to be expected with SGLT2is on MACEs, hospitalisation for HF and progression of renal disease depending on the baseline characteristics of the patients with T2DM, i.e., patients in secondary versus primary prevention of ASCVD (12).
These positive results were confirmed in a CVOT that recruited patients with T2DM and albuminuric CKD, all already treated with blockers of the renin-angiotensin system. In the CREDENCE trial, recently published in the New England Journal of Medicine (13), all the patients had an eGFR of 30 to <90 mL/min/1.73 m2 and a ratio of albumin to creatinine >300 to 5,000 mg/g. After a median follow-up of 2.62 years (early interruption recommended by the data and safety monitoring committee), the relative risk of the primary outcome [a composite of end-stage renal disease (ESRD: dialysis, transplantation, or a sustained eGFR of <15 mL/min/1.73 m2), a doubling of the serum creatinine concentration, or death from renal or cardiovascular causes] was 30% lower with canagliflozin than with placebo [hazard ratio or HR, 0.70; 95% confidence interval (CI), 0.59 to 0.82; P=0.00001]. The relative risk of the renal-specific composite endpoint (excluding cardiovascular death) was also lower by 34% (HR, 0.66; 95% CI, 0.53 to 0.81; P<0.001), and the relative risk of ESRD was lower by 32% (HR, 0.68; 95% CI, 0.54 to 0.86; P=0.002). Patients treated with canagliflozin also had a lower risk of 3-point MACEs (HR, 0.80; 95% CI, 0.67 to 0.95; P=0.01) and hospitalization for HF (HR, 0.61; 95% CI, 0.47 to 0.80; P<0.001) (13). Thus, CREDENCE specifically dedicated to patients with T2DM and albuminuric CKD confirms prespecified exploratory results of previous CVOTs summarized in the meta-analysis by Zelniker and colleagues (11). Indeed, this trial further demonstrates that SGLT2is improve renal outcomes (reduced risk of kidney failure) and cardiovascular outcomes (less MACEs) in patients with T2DM and CKD, and these effects were already seen after a rather short median follow-up of about 2.6 years in this high-risk population (13).
When comparing the results obtained in different CVOTs, SGLT2is (gliflozins) exhibited obvious advantages compared with dipeptidyl peptidase-4 inhibitors (gliptins), as the latter glucose-lowering agents showed only non-inferiority versus placebo, without superiority (14). When comparing SGLT2is with glucagon-like peptide-1 receptor agonists (GLP-1RAs), the reduction in the primary composite endpoint (3-point MACEs) was almost similar. This is the case when comparing the results from EMPA-REG OUTCOME with empagliflozin (8) with those from the LEADER trial with liraglutide (15), yet the difference occurs much earlier with the SGLT2i than with the GLP-1RA. Furthermore, results appear more heterogeneous within the GLP-1RA pharmacological class than among SGLT2is (16). However, a detailed analysis of the individual components of 3-point MACEs showed that the cardiovascular benefits of GLP-1RAs are predominantly on ASCVD events (non-fatal myocardial infarction, non-fatal stroke and CV death). In contrast, SGLT2is have less effects on ASCVD events (non-fatal myocardial infarction/stroke). Their benefits are predominantly on hospitalization for HF and cardiovascular death, which suggest effects primarily on myocardial function (the “pump”), and not on coronary arteries (the “pipes”) (12,17). Indeed, the protective effects of SGLT2is on hospitalisation for HF (11) were clearly superior compared to those reported with GLP-1RAs (18). Results regarding renoprotective effects seem also better with SGLT2is than with GLP-1RAs, with a significant reduction in hard clinical endpoints including progression to ESRD with SGLT2is rather than a reduction mainly in surrogate endpoints such as albuminuria with GLP-1RAs (19).
These important results reported in the different CVOTs for the last five years have markedly influenced the recent consensus report by experts from the American Diabetes Association and the European Association for the Study of Diabetes (20). Indeed, a systematic evaluation of the literature since 2014 (but before the official publication of DECLARE-TIMI 58) informed new recommendations. Among patients with T2DM who have established ASCVD, SGLT2is or GLP-1RAs with proven cardiovascular benefit are recommended as part of glycemic management. Among patients with ASCVD in whom HF coexists or is of special concern, SGLT2is are preferably recommended. For patients with T2DM and CKD, with or without ASCVD, it is recommended to consider the use of an SGLT2i shown to reduce CKD progression (provided eGFR is adequate), or, if contraindicated or not preferred, a GLP-1RA shown to reduce CVD and perhaps CKD progression. Thus, the selection of medication added to metformin should be based on patient preference and clinical profile. Important patient characteristics include the presence of established ASCVD and other comorbidities such as HF or CKD. The risk for specific adverse events related to the medication, particularly hypoglycemia and weight gain, as well as overall safety, tolerability, patient preference and cost may also influence the medication choice, especially in the decision to choose between an SGLT2is and a GLP-1RA (20).
A 2018 report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways confirmed this treatment algorithm in patients with T2DM and ASCVD (21). Of note, the patients represented in the three CVOTs discussed by Zelniker and colleagues (11) all had, or were at high risk for, ASCVD. Whether similar cardiovascular outcome effects would occur in lower risk patients without established ASCVD is not known. A report from a roundtable organized by the European Society of Cardiology addressing cardiovascular risk in patients with T2DM (22) endorsed the conclusion of the ADA-EASD 2918 consensus report in patients with ASCVD (20). However, it also mentioned that in view of the reduction of MACEs and cardiovascular death, physicians need to consider prioritization of glucose-lowering agents such as SGLT2is and GLP-1-RAs that have proven cardiovascular protection much earlier in the management of T2DM. Indeed, there is no clear evidence to indicate that a different response should occur in treatment-naïve patients (i.e., without metformin as background therapy) or those with glycated haemoglobin (HbA1c) <7% (53 mmol/mol) (22). A detailed analysis of available results from published CVOTs with SGLT2is or GLP-1RAs, comparing CV outcomes in T2DM patients with low versus high HbA1c levels, led to the conclusion that there is no reason not to add a glucose-lowering agent with proven cardioprotection in high-risk patients with T2DM despite they are at HbA1c target on metformin (23).
Zelniker and colleagues (11) concluded that SGLT2is have moderate benefits on atherosclerotic MACEs that seem confined to patients with established ASCVD. However, a 2019 report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines on the primary prevention of cardiovascular disease concluded as follows: “For adults with T2DM and additional ASCVD risk factors who require glucose-lowering therapy despite initial lifestyle modifications and metformin, it may be reasonable to initiate an SGLT2i or a GLP-1RA to improve glycemic control and reduce CVD risk (class of recommendation IIB; level of evidence B-R)” (24).
We are currently facing an exciting time period for the management of hyperglycemia in T2DM, with the publication of results of numerous CVOTs, which already have but also will have a major impact on guidelines. The next CVOT in patients with T2DM and established ASCVD (a population close to that of EMPA-REG OUTCOME) will be VERTIS-CV comparing ertugliflozin versus placebo, whose results are expected within the next year (Table 2). The results of other ongoing trials with SGLT2is, especially those focusing on patients with HF (with preserved or reduced left ventricular ejection fraction) or with CKD are awaited with great interest by the medical community, not only endocrinologists, but also cardiologists and nephrologists (Table 2) (25). Of major interest, some of these trials will include patients with and without T2DM. We are waiting for a confirmation of the secondary analyses regarding the risk of hospitalisation for HF and of progression of renal disease reported in the three initial CVOTs in patients with T2DM and high risk of ASCVD analysed by Zelniker and colleagues (11). If confirmed, such findings obviously will open new perspectives for the management of patients with HF or CKD, not only for patients with T2DM but possibly also for patients without T2DM (25). This will be a strange and indeed unexpected issue for drugs that were initially developed as glucose-lowering agents!

Full table
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 2015;75:33-59. [Crossref] [PubMed]
- DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 2017;13:11-26. [Crossref] [PubMed]
- Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: potential contribution for diabetic complications and cardiovascular disease. Diabetes Metab 2018;44:457-64. [Crossref] [PubMed]
- Scheen AJ. An update on the safety of SGLT2 inhibitors. Expert Opin Drug Saf 2019;18:295-311. [Crossref] [PubMed]
- Scheen AJ. SGLT2 inhibitors: benefit/risk balance. Curr Diab Rep 2016;16:92. [Crossref] [PubMed]
- Lupsa BC, Inzucchi SE. Use of SGLT2 inhibitors in type 2 diabetes: weighing the risks and benefits. Diabetologia 2018;61:2118-25. [Crossref] [PubMed]
- Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a Diabetes Care Editors' Expert Forum. Diabetes Care 2018;41:14-31. [Crossref] [PubMed]
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117-28. [Crossref] [PubMed]
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644-57. [Crossref] [PubMed]
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347-57. [Crossref] [PubMed]
- Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31-9. [Crossref] [PubMed]
- Verma S, Juni P, Mazer CD. Pump, pipes, and filter: do SGLT2 inhibitors cover it all? Lancet 2019;393:3-5. [Crossref] [PubMed]
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295-306. [Crossref] [PubMed]
- Scheen AJ. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 inhibitors. Circ Res 2018;122:1439-59. [Crossref] [PubMed]
- Scheen AJ. Cardiovascular outcome studies in type 2 diabetes: comparison between SGLT2 inhibitors and GLP-1 receptor agonists. Diabetes Res Clin Pract 2018;143:88-100. [Crossref] [PubMed]
- Scheen AJ. GLP-1 receptor agonists and cardiovascular protection. Class effect or not? Diabetes Metab 2018;44:193-6. [Crossref] [PubMed]
- Hupfeld C, Mudaliar S. Navigating the "MACE" in cardiovascular outcomes trials and decoding the relevance of atherosclerotic CVD benefits versus heart failure benefits. Diabetes Obes Metab 2019;21:1780-9. [PubMed]
- Scheen AJ. GLP-1 receptor agonists and heart failure in diabetes. Diabetes Metab 2017;43 Suppl 1:2S13-2S9.
- Scheen AJ. Effects of glucose-lowering agents on renal surrogate endpoints and hard clinical outcomes in patients with type 2 diabetes. Diabetes Metab 2019;45:110-21. [Crossref] [PubMed]
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669-701. [Crossref] [PubMed]
- Das SR, Everett BM, Birtcher KK, et al. 2018 ACC Expert Consensus Decision Pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: A report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;72:3200-23. [Crossref] [PubMed]
- Cosentino F, Ceriello A, Baeres FM, et al. Addressing cardiovascular risk in type 2 diabetes mellitus: a report from the European Society of Cardiology Cardiovascular Roundtable. Eur Heart J 2018. [Epub ahead of print].
- Scheen AJ. Why not adding a glucose-lowering agent with proven cardioprotection in high-risk patients with type 2 diabetes at HbA1c target on metformin? Diabetes Res Clin Pract 2019;147:169-71. [Crossref] [PubMed]
- Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019.2019. [Epub ahead of print].
- Scheen AJ. Series: Implications of the recent CVOTs in type 2 diabetes. Impact on guidelines: the endocrinologist point of view Diabetes Res Clin Pract 2019. [Crossref] [PubMed]


