
Paraneoplastic syndromes in lung cancer and their management
Introduction
Paraneoplastic syndromes refer to the remote effects associated with malignancy which are unrelated to direct tumor invasion or metastases (1). These may occur before the cancer is diagnosed and can be independent in their severity to the stage of the primary tumor. Paraneoplastic syndromes are most commonly associated with lung cancer, reported in approximately 10% of cases (1). Endocrine syndromes, particularly syndrome of inappropriate ADH secretion (SIADH) and humoral hypercalcemia of malignancy (HHM) are the most common paraneoplastic syndromes seen in lung cancer and are related to the histologic type of cancer (1). This review considers a variety of paraneoplastic syndromes associated with lung cancer and discusses their pathophysiology, clinical features and management options.
Endocrine syndromes
Hypercalcemia
Hypercalcemia has been reported in 2–6% lung cancer patients at the time of initial diagnosis; the incidence increases to 8–12% over the disease course (2). It is associated with a poor prognosis. When associated with PTHrP production (parathyroid hormone related-protein), it is referred to as HHM.
HHM is observed in a variety of malignancies such as breast, renal, multiple myeloma and lung; squamous cell is the most frequently observed subtype (3-5). Osteolytic metastases are another significant cause of hypercalcemia in malignancy.
Of the four mechanism of hypercalcemia secondary to HHM (secretion of PTHrP, parathyroid hormone, 1-25 dihydroxy vitamin D or granulocyte colony stimulating factor), secretion of parathyroid hormone related protein is the most common in lung cancer. Parathyroid hormone production is reported as a rare mechanism (6). Chronic G-CSF exposure promotes osteoclastic bone resorption (7).
HHM is typically found in advanced disease and is associated with a poor prognosis (7). The clinical features of hypercalcemia are variable and can be non-specific; gastrointestinal symptoms (such as nausea, vomiting abdominal pain and constipation) are common. Dehydration (vomiting, polyuria) in the setting of hypercalcemia can result in renal dysfunction (3-5). Neuropsychiatric manifestations range from fatigue and lethargy to changes in mood and cognitive dysfunction (3-5).
Diagnostic evaluation should ideally ionized calcium measurement in addition to PTH levels. However, if ionized calcium levels are not available, then calcium and albumin levels should be measured at the same time and calcium levels should be corrected for albumin levels. As for most paraneoplastic endocrine syndromes, treatment of the underlying malignancy is the most successful treatment strategy (3). Acute hypercalcemia is managed with intravenous fluid administration (3) with frequent monitoring of calcium levels. Loop decrease calcium reabsorption and can be added after adequate fluid resuscitation. Bisphosphonates are another useful treatment option due to their inhibitory effects on bone resorption (3). Calcitonin also suppresses bone resorption and is useful in the short term (4,5). Denosumab binds to RANKL to prevent ligand interaction with RANK receptors on precursor osteoclasts which interferes with osteoclast maturation and survival (8). It prevents skeletal-related events in patients with bone metastases and is a generally well-tolerated treatment (9).
SIADH
SIADH represents a state of euvolemic, hypoosmolar hyponatremia, which, in the case of lung cancer is secondary to ectopic ADH production; 10–45% of small cell lung cancer (compared to 1% of non-small lung cancers) can produce ectopic ADH (10,11) resulting in excessive urinary sodium excretion. Hypothyroidism, volume depletion and adrenal insufficiency should be excluded. Although the stage of SCLC does not seem to affect the occurrence of SIADH, hyponatremia tends to worsen prognosis in comparison to normal sodium levels in these patients (12). The symptoms of SIADH depend on the degree and acuity of the hyponatremia. Non-specific symptoms such as headache and fatigue might be the initial presentation of paraneoplastic SIADH. Acute (<48 hours), severe (serum sodium <120 meq/L) hyponatremia leads to cerebral edema causing altered mental status, seizures and death. Chronic, mild to moderate, hyponatremia may not produce any significant neurologic symptoms.
Definitive management of paraneoplastic SIADH involves treatment of the malignancy itself. Chemotherapy for SCLC can mitigate and in several cases resolve SIADH in at least 80% of patients in some studies (4). Recurrence of SIADH can be related to tumor recurrence or progression (13). Beyond treatment of the cancer, paraneoplastic SIADH is managed largely in the same way as it is in patients without cancer.
In acute, severe hyponatremia, particularly with neurologic symptoms, hypertonic saline is administered. Rapid overcorrection is avoided because of the risk of osmotic demyelination. In milder, asymptomatic cases, free water restriction (1 L/day) is the first step. Pharmacologic treatments may be employed if conservative measures fail to produce an adequate response. Demeclocycline, a tetracycline antibiotic, decreases renal response to ADH and has been used to manage SIADH. Vasopressin receptor antagonists (conivaptan, tolvaptan) increase urinary free water excretion and have also been shown to be effective.
Ectopic Cushing’s syndrome (ECS)
The manifestations of ECS result from the unregulated production of adrenocorticotropic hormone (ACTH) from malignant cells (4). Elevated ACTH levels may be detectable in up to 50% of patients with lung cancer (13,14). Ectopic ACTH secretion is almost always associated with SCLC (14) or less commonly bronchial carcinoids. About 30% of all cases of SCLC are associated with ectopic hyper-secretion of ACTH (15), and this tends to confer a worse prognosis (16).
ACTH stimulates the adrenal production of glucocorticoids (17). Hypercortisolism results in hypokalemic metabolic alkalosis and secondary hypertension that is difficult to treat. Hyperglycemia is also common (18,19). Characteristic findings on physical examination include abnormal fat deposition (moon facies, buffalo hump, centripetal obesity) and violaceous striae (20).
The diagnosis of ECS requires the exclusion of iatrogenic hypercortisolism from exogenous steroids (21). Initial work-up of hypercortisolism includes measurements of late-night salivary cortisol, 24-hour urinary cortisol and the 1mg overnight dexamethasone suppression test. If results suggest hypercortisolism, an ACTH level can distinguish Cushing’s from ECS (22). The absence of a pituitary tumor on imaging (CT, MRI) with an elevated morning ACTH raises the suspicion of ECS. High-dose dexamethasone will suppress a pituitary but not an ectopic source of ACTH.
In most cases, treatment of the underlying cancer will also improve the paraneoplastic syndrome. Ideal treatment is to excise the tumor (23). A number of medications (such as ketoconazole and metyrapone) decrease cortisol synthesis (19) and can be used prior to definitive management with surgery or chemoradiotherapy.
Acromegaly
Ectopic growth hormone releasing hormone (GHRH) secretion from malignant cells can manifest as acromegaly (24); in the case of lung cancer, bronchial carcinoids (25) and epidermoid carcinomas (26) have been implicated; SCLC have been reported less frequently (27,28). Surgical removal of the primary tumor is usually the most successful treatment option (29,30). Somatostatin analogs suppress growth hormone release and are another management option (31,32).
Pulmonary carcinoid syndrome
Bronchopulmonary neuroendocrine tumors (BP-NET) comprise approximately 20% of all lung cancers (33). Less than 5% of patients with BP-NET present with carcinoid syndrome (33). Lung NET produce less serotonin than midgut NET accounting for a lower rate of carcinoid syndrome (33). Patients with a Lung NET who have carcinoid syndrome may have atypical symptoms such as episodes of flushing which are excessively prolonged (34). The specific hormone mediator of flushing in patients is unclear in these patients. In some cases, blood serotonin or urine 5-hydroxyindoleacetic acid (5-HIAA) levels are normal. The risk of carcinoid crisis is low in these patients and prophylactic octreotide is not recommended by most clinicians prior to tumor manipulation (34).
Neurological syndromes
Paraneoplastic neurological syndromes (PNSs) are autoimmune in nature; unlike most paraneoplastic syndromes, they are independent of local tumor or metastatic effects (35). Onconeural antibodies appear to be central to the pathogenesis, though their absence does not preclude a diagnosis of PNS (36). These antibodies are directed against tumor cells but can also target the nervous system (central and peripheral), resulting in the wide-ranging manifestations of PNS.
In cases of possible or definite PNS all other possible causes of neurological causes should be excluded like brain or leptomeningeal metastasis, nerve root or spinal cord invasion or compression, electrolyte disturbances, hyperglycemia, or adverse effects to irradiation or chemotherapy.
A number of onconeural antibodies have been identified, such as Anti-Hu, Anti-CV2, anti-amphiphysin and Anti-Ri (2). Anti-Hu antibodies are the most commonly detected and 90% of Anti-Hu syndrome cases are seen in SCLC (2). The term Anti-Hu syndrome is broad, and encompasses several entities including limbic encephalitis (LE) (37), cerebellar degeneration, opsoclonus-myoclonus, neuropathies and gastric pseudo-obstruction. Some of these are discussed in detail here along with various treatment modalities.
LE
LE has is characterized by acute or sub-acute neuropsychiatric symptoms including changes in mood, memory, seizures and cognitive function (37). Symptoms typically progress over days to months. Characteristic EEG and MRI findings can support the diagnosis.
SCLC is the most commonly associated malignancy, with most patients being anti-Hu antibody positive. In addition to treatment of the malignancy, paraneoplastic LE can respond, sometimes dramatically so to immunotherapy (38).
Lambert-Eaton myasthenia syndrome (LEMS)
LEMS is a disorder of the neuromuscular junction resulting from decreased pre-synaptic acetylcholine release. Nearly half have an associated malignancy (38). The basis is autoimmune and antibodies directed against the voltage-gated calcium channel (VGCC) are usually involved in paraneoplastic LEMS. Paraneoplastic LEMS is almost invariably associated with SCLC (38); autoantibodies target the VGCCs expressed on the surface of tumor cells. This results in decreased acetylcholine release and inhibition of synaptic conduction.
The hallmark of LEMS is proximal muscle weakness, predominantly affecting the lower extremities (starting from the pelvic girdle) (39). Upper extremity involvement is usually milder and weakness can progress in a craniocaudal direction (39). Muscle weakness in the setting of SCLC in addition to characteristic electromyography (EMG) changes and the presence of autoantibodies are supportive of the diagnosis. Symptoms improve with treatment of the underlying malignancy (40). Targeted therapy for symptomatic LEMS with 3,4-diaminopyridine is usually first line; the use of guanidine with or without acetylcholinesterase inhibitors (40) is limited by marrow suppression and nephrotoxicity. Steroids, intravenous immunoglobulin (IVIg), immunosuppressants and plasma exchange are options for refractory cases (41).
Subacute cerebellar degeneration (SCD)
Paraneoplastic SCD is rare and most commonly associated with SCLC. Autoantibodies directed at the cerebellum, particularly the Purkinje cells are involved in the pathogenesis (37). In contrast to anti-Hu associated PNSs, SCD is usually associated with anti-Yo, anti-Tr and anti-metabotropic glutamate receptor 1 (mGluR1) antibodies. Radiographic evidence of cerebellar atrophy is initially absent (37) but can develop as the disease progresses.
Symptoms of cerebellar dysfunction such as nausea, vomiting, vertigo, gait instability and ataxia are typically present. Prognosis is usually poor and immunotherapy results in modest improvement at best (37); disease progression and permanent neurologic disability is often seen.
Subacute sensory neuropathy (SSN)
Paraneoplastic SSN is a constellation of neurologic symptoms beginning with the loss of vibratory and joint sense and progressing, usually within 12 weeks, to impaired temperature sensation and pain. The pain is described as shock-like and ataxia is common. Symptoms can involve all 4 extremities, often asymmetrically (37). SCLC is the most commonly associated malignancy. Electrophysiology demonstrates significant sensory fiber disturbance, minimal motor nerve involvement, depressed or absent tendon reflex and often autonomic, cerebellar, or cerebral abnormalities (37).
Prompt recognition and treatment of the malignancy offers the best chance of improvement in neurologic symptoms (35). Compared with other PNSs, response to glucocorticoids, IVIg, plasma exchange and immunosuppressants is poor, though there is some evidence that combination treatments may result in better outcomes (41).
Glomerular diseases
In 1966, Lee et al. (42) reported an increased incidence of nephrotic syndrome in patients with malignancies. Since then, several cases of paraneoplastic glomerulopathy have been reported. Lung cancer is usually associated with paraneoplastic nephrotic syndromes, the most common of which is membranous glomerulopathy (MGN) (43). However, cases of minimal change disease (MCD), IgA Nephropathy, Membranoproliferative glomerulonephritis (MPGN), Focal segmental glomerulosclerosis among others have also been reported.
Nephrotic syndrome usually manifests either before or at the time of cancer diagnosis; less frequently, it occurs after the diagnosis (44). The pathogenesis might involve host-antibody response to tumor antigen shedding (45); these complexes might suppress the antineoplastic effects of cytotoxic lymphocytes (46). The detection of these antibodies or complexes is usually not necessary for the diagnosis; excluding alternate etiologies and improvement in the nephrotic syndrome associated with treatment of the malignancy can point towards the diagnosis.
Surgical resection of the primary tumor has been shown in some cases to resolve paraneoplastic nephrotic syndrome (47,48). In the case of advanced disease or unresectable lung cancer, treatment is less clear. Chemotherapy and radiotherapy have also been used successfully, for both SCLC and advanced NSCLC (48); tumor progression, however, lead to the recurrence of renal disease (48). Carboplatin based regimens are preferentially used, given the nephrotoxicity of cisplatin.
MCD is a smaller proportion of paraneoplastic glomerulopathy; though usually seen in conjunction with Hodgkin’s Lymphoma, cases with lung cancer have also been reported. Treatment of the primary tumor and early use of steroids are the cornerstones of the management of paraneoplastic MCD.
Hematologic syndromes
Hypercoagulability
Venous thromboembolism (VTE)
VTE including DVT, PE and superficial vein thrombosis occurs in nearly 3% of lung cancer patients within the first 2 years of diagnosis (49). Patients with lung cancer have a 20-fold increase in risk of VTE compared to the general population (49). NSCLC confers a higher VTE risk than SCLC, and adenocarcinomas are associated with a higher risk of VTE than squamous cell carcinoma (3,50). Distant metastases confer a fold increase in VTE compared to localized tumors (51). More so, tissue factor (TF), which initiates the coagulation cascade and cancer procoagulant have an increased expression in lung cancer cells (52). TF-bearing microparticles, possibly originating from malignant cells themselves, may also contribute to a prothrombotic state.
Treatment of cancer-associated venous thromboembolic disease depends on a number of factors, including medical comorbidities (renal, hepatic disease), drug interactions, bleeding risk and reversibility, setting (inpatient/outpatient), compliance and cost. The NCCN (National Comprehensive Cancer Network) has developed guidelines (year 2018) to guide clinical decision-making. Single-agent LMWH is the preferred therapeutic anticoagulation option for cancer-related VTE (NCCN) (53) Randomized trials have shown LMWH to have an equal or decreased risk of VTE compared with LMWH plus VKA (vitamin K antagonist) combination regimens (53). More so, the risk of major bleeding and survival rates are comparable between the mono and combination therapy groups. LMWH has consistently been shown to be superior to VKA in the prevention of recurrent cancer-related VTE (53). Trials for LMWH and VKA did not compare the 2 for a duration longer than 6 months. Among the LMWHs, the efficacy of dalteparin is supported by the highest-quality evidence for cancer-related VTE (53). LMWHs must be used cautiously in patients with renal dysfunction.
Among the DOACs (direct-acting oral anticogaulants), rivaroxaban was shown to have similar or better efficacy than dalteparin, with a slightly increased bleeding risk and equal survival rates at 6 months (53,54). Combination treatment with initial LMWH followed by longer-term edoxaban is an alternate regimen, though the risk of bleeding was significantly higher in the combination regimen (Hokusai-VTE Cancer). Randomized trials comparing apixaban with LMWH for the treatment of cancer-related VTE are underway. DOACs are contraindicated in patients with Stage IV chronic kidney disease and active or clinically significant liver disease. DOACs have been associated with increased bleeding risk in patients with gastrointestinal or genitourinary lesions, surgery or instrumentation and therefore must be used cautiously in these groups (51,54).
Recently, results from the CASSINI Trial, showed that primary prophylaxis with rivaroxaban reduced the risk of VTE and VTE-related deaths in high-risk cancer patients (55). Apixaban was also shown to be beneficial in reducing risk of VTE in intermediate to high-risk cancer patients. Both studies did result in more bleeding episodes in the anticoagulation group as compared to placebo.
Trousseau’s syndrome
‘Trousseau’s syndrome’ originally described migratory thrombophlebitis occurring in association with malignancy, but the term has also been used in reference to cancer-related hypercoagulability syndromes (56). The pathogenesis is complex, but possibly involves factor X-mediated intravascular coagulation, as observed in studies involving adenocarcinomas (57). LMWH is typically used to manage the various manifestations of Trousseau’s syndrome (58). Warfarin does not appear to be as effective, possibly because it does not target selectin-binding (unlike heparin), which might a central process in the pathogenesis (59). There is no evidence for the role of anti-platelet agents.
Rheumatologic syndromes
Hypertrophic pulmonary osteoarthropathy (HPO)
HPO is the proliferation of distal cutaneous and osseous tissues (60,61) resulting in clubbing of the fingers and toes, symmetric painful arthropathy and long bone periostosis. HPO is most frequently associated with lung cancer (62,63). Unlike rheumatoid arthritis, there are no erosions or inflammatory synovitis, and there is no joint space narrowing as seen in osteoarthritis (64). Bone scintigraphy can show periosteal thickening, typically in the tibiae and fibulae (62,63). The pathophysiology of HPO is poorly understood, but it may involve overexpression of vascular endothelial growth factor (VEGF) (65) and PDGF.
Treatment of the primary malignancy has been shown to resolve HPO (62). Targeted therapy for gefitinib has also been used (66). Additionally, there have been reports of bisphosphonates, particularly pamidronate with potential anti-VEGF properties (67), and octreotide providing significant symptomatic relief (68).
Inflammatory myopathies
Polymyositis (PM) and dermatomyositis (DM) are autoimmune inflammatory myopathies sometimes seen in the setting of malignancy. They are characterized by proximal, painless muscle weakness; dermatomyositis is associated with a heliotrope rash (purple periorbital discoloration), Gottron papules (purple papules and plaques on the dorsum of both hands) and less frequently photosensitivity (69) (Figure 1).
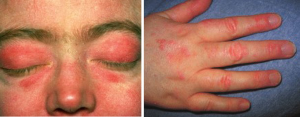
DM is associated with malignancy in 10–40% of cases and often arises within 1 year of cancer diagnosis (70,71). While more commonly seen with nasopharyngeal, breast and ovarian cancers, an association with lung cancer has been observed (72,73). SCLC is more commonly associated with DM than squamous cell carcinoma (74).
High-dose oral glucocorticoid therapy is the first-line medical treatment for inflammatory myopathies and should be considered in addition to addressing the underlying malignancy (74,75). Methotrexate/azathioprine or rituximab can be considered for refractory disease.
Paraneoplastic dermatologic syndromes
Acanthosis nigricans (AN)
AN is the thickening and hyperpigmentation of the skin in intertriginous regions (neck folds, axilla) (3). Oral lesions are less common. Although AN is associated with endocrinopathies, a paraneoplastic variant is also seen (3). Paraneoplastic AN is commonly associated with intra-abdominal tumors and less commonly with NSCLC (76,77). Some patients develop concurrent ‘tripe palms,’ with velvety, rugose thickening of the palms and less commonly the soles which are also associated with malignancy (78). In some cases, tripe palms are the presenting feature of an underlying malignancy. Abnormalities in insulin-like growth factor (IGFR) receptors and fibroblasts have been observed in these cutaneous syndromes. AN and tripe palms typically improve significantly with treatment of the underlying malignancy (79) and topical retinoids are also useful.
Conclusions
This review aimed to summarize current perspectives on paraneoplastic syndromes associated with lung cancer and their management. Treating the underlying cancer is most likely to improve the effects of paraneoplastic syndromes. Greater research into their underlying mechanisms is being used to develop targeted therapies.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Heinemann S, Zabel P, Hauber HP. Paraneoplastic syndromes in lung cancer. Cancer Ther 2008;6:687-98.
- Spiro SG, Gould MK, Colice GL. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes: aCCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:149S-60S.
- Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc 2010;85:838-54. [Crossref] [PubMed]
- Mazzone PJ, Arroliga AC. Endocrine paraneoplastic syndromes in lung cancer. Curr Opin Pulm Med 2003;9:313-20. [Crossref] [PubMed]
- Clines GA. Mechanisms and treatment of hypercalcemia of malignancy. Curr Opin Endocrinol Diabetes Obes 2011;18:339-46. [Crossref] [PubMed]
- Yoshimoto K, Yamasaki R, Sakai H, et al. Ectopic production of parathyroid hormone by small cell lung cancer in a patient with hypercalcemia. J Clin Endocrinol Metab 1989;68:976-81. [Crossref] [PubMed]
- Hiraki A, Ueoka H, Takata I, et al. Hypercalcemia-leukocytosis syndrome associated with lung cancer. Lung Cancer 2004;43:301-7. [Crossref] [PubMed]
- Hanley DA, Adachi JD, Bell A, et al. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract 2012;66:1139-46. [Crossref] [PubMed]
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010;28:5132-9. [Crossref] [PubMed]
- Kagawa K, Fujitaka K, Isobe T, et al. Syndrome of inappropriate secretion of ADH (SIADH) following cisplatin administration in a pulmonary adenocarcinoma patient with a malignant pleural effusion. Intern Med 2001;40:1020-3. [Crossref] [PubMed]
- Moses AM, Scheinman SJ. Ectopic secretion of neurohypophyseal peptides in patients with malignancy. Endocrinol Metab Clin North Am 1991;20:489-506. [Crossref] [PubMed]
- Hansen O, Sørensen P, Hansen KH. The occurrence of hyponatremia in SCLC and the influence on prognosis: a retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer 2010;68:111-4. [Crossref] [PubMed]
- List AF, Hainsworth JD, Davis BW, et al. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in small-cell lung cancer. J Clin Oncol 1986;4:1191-8. [Crossref] [PubMed]
- Mendelsohn G, Baylin SB. Ectopic hormone production: biological and clinical implications. Prog Clin Biol Res 1984;142:291-316. [PubMed]
- Mennecier B, Moreau L, Goichot B, et al. Paraneoplastic Cushing’s syndrome and small cell bronchial carcinoma. Rev Pneumol Clin 1999;55:77-80. [PubMed]
- Terzolo M, Reimondo G, Ali A, et al. Ectopic ACTH syndrome: molecular basis and clinical heterogeneity. Ann Oncol 2001;12:S83-7. [Crossref] [PubMed]
- Shepherd FA, Laskey J, Evans WK, et al. Cushing’s syndrome associated with ectopic corticotropin production and small-cell lung cancer. J Clin Oncol 1992;10:21-7. [Crossref] [PubMed]
- Delisle L, Boyer MJ, Warr D, et al. Ectopic corticotropin syndrome and small-cell carcinoma of the lung. Clinical features, outcome, and complications. Arch Intern Med 1993;153:746-52. [Crossref] [PubMed]
- Ost DE, Jim Yeung SC, Tanoue LT, et al. Clinical and organizational factors in the initial evaluation of patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e121S-41S.
- Guignat L, Bertherat J. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline: commentary from a European perspective. Eur J Endocrinol 2010;163:9-13. [Crossref] [PubMed]
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008;93:1526-40. [Crossref] [PubMed]
- Invitti C, Pecori Giraldi F, de Martin M, et al. Diagnosis and management of Cushing’s syndrome: results of an Italian multicenter study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. J Clin Endocrinol Metab 1999;84:440-8. [PubMed]
- Alexandraki KI, Grossman AB. The ectopic ACTH syndrome. Rev Endocr Metab Disord 2010;11:117-26. [Crossref] [PubMed]
- Faglia G, Arosio M, Bazzoni N. Ectopic acromegaly. Endocrinol Metab Clin North Am 1992;21:575-95. [Crossref] [PubMed]
- Biswal S, Srinivasan B, Dutta P, et al. Acromegaly caused by ectopic growth hormone: a rare manifestation of a bronchial carcinoid. Ann Thorac Surg 2008;85:330-2. [Crossref] [PubMed]
- El Aziz S, Chadli A, Obbiba A, et al. Pulmonary epidermoid carcinoma in a patient with acromegaly: a rare entity. Pan Afr Med J 2012;12:27. [PubMed]
- Melmed S. Extrapituitary acromegaly. Endocrinol Metab Clin North Am 1991;20:507-18. [Crossref] [PubMed]
- Doga M, Bonadonna S, Burattin A, et al. Ectopic secretion of growth hormone-releasing hormone (GHRH) in neuroendocrine tumors: relevant clinical aspects. Ann Oncol 2001;12 Suppl 2:S89-94. [Crossref] [PubMed]
- Athanassiadi K, Exarchos D, Tsagarakis S, et al. Acromegaly caused by ectopic growth hormone-releasing hormone secretion by a carcinoid bronchial tumor: a rare entity. J Thorac Cardiovasc Surg 2004;128:631-2. [Crossref] [PubMed]
- Gudbjartsson T, Agnarsson BA, Palsson PS, et al. Acromegaly caused by ectopic growth hormone-releasing hormone production from a bronchial carcinoid tumor. Thorac Cardiovasc Surg 2011;59:184-5. [Crossref] [PubMed]
- Drange MR, Melmed S. Long-acting lanreotide induces clinical and biochemical remission of acromegaly caused by disseminated growth hormone-releasing hormone-secreting carcinoid. J Clin Endocrinol Metab 1998;83:3104-9. [Crossref] [PubMed]
- Butler PW, Cochran CS, Merino MJ, et al. Ectopic growth hormone-releasing hormone secretion by a bronchial carcinoid tumor: clinical experience following tumor resection and long-acting octreotide therapy. Pituitary 2012;15:260-5. [Crossref] [PubMed]
- Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5. [Crossref] [PubMed]
- Thomas CF, Jett JR, Strosberg JR, et al. Lung neuroendocrine (carcinoid) tumors: Epidemiology, risk factors, classification, histology, diagnosis, and staging. Available online: https://www.uptodate.com/contents/lung-neuroendocrine-carcinoid-tumors-epidemiology-risk-factors-classification-histology-diagnosis-and-staging
- Yeung SC, Habra MA, Thosani SN. Lung cancer-induced paraneoplastic syndromes. Curr Opin Pulm Med 2011;17:260-8. [Crossref] [PubMed]
- Honnorat J, Antoine JC. Paraneoplastic neurological syndromes. Orphanet J Rare Dis 2007;2:22. [Crossref] [PubMed]
- Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135-40. [Crossref] [PubMed]
- Gultekin SH, Rosenfeld MR, Voltz R, et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumor association in 50 patients. Brain 2000;123:1481-94. [Crossref] [PubMed]
- Titulaer MJ, Maddison P, Sont JK, et al. Clinical Dutch-English Lambert-Eaton Myasthenic syndrome (LEMS) tumor association prediction score accurately predicts small-cell lung cancer in the LEMS. J Clin Oncol 2011;29:902-8. [Crossref] [PubMed]
- Pourmand R. Lambert Eaton myasthenic syndrome. Front Neurol Neurosci 2009;26:120-5. [Crossref] [PubMed]
- Feasby T, Banwell B, Benstead T, et al. Guidelines on the use of intravenous immune globulin for neurologic conditions. Transfus Med Rev 2007;21:S57-107. [Crossref] [PubMed]
- Lee JC, Yamauchi H, Hopper J Jr. The association of cancer and the nephrotic syndrome. Ann Intern Med 1966;64:41-51. [Crossref] [PubMed]
- Lin FC., Chen J Y, Yang AH, et al. The association of non-small-cell lung cancer, focal segmental glomerulosclerosis, and platelet dysfunction. Am J Med Sci 2002;324:161-5. [Crossref] [PubMed]
- Burstein DM, Korbet SM, Schwartz MM. Membranous glomerulonephritis and malignancy. Am J Kidney Dis 1993;22:5-10. [Crossref] [PubMed]
- Eagen JW, Lewis EJ. Glomerulopathies of neoplasia. Kidney Int 1977;11:297-303. [Crossref] [PubMed]
- Coltharp WH, Lee SM, Miller RF, et al. Nephrotic syndrome complicating adenocarcinoma of the lung with resolution after resection. Ann Thorac Surg 1991;51:308-9. [Crossref] [PubMed]
- Pauker SG, Kopelman RI. Hunting for the cause: how far to go? N Engl J Med 1993;328:1621-4. [Crossref] [PubMed]
- Yangui I, Msaad S, Smaoui M, et al. Small-cell lung cancer and rapidly fatal nephrotic syndrome. Rev Pneumol Clin 2007;63:331-4. [Crossref] [PubMed]
- Chew HK, Davies AM, Wun T, et al. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost 2008;6:601-8. [Crossref] [PubMed]
- Blom JW, Osanto S, Rosendaal FR. The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost 2004;2:1760-5. [Crossref] [PubMed]
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018;378:615-24. [Crossref] [PubMed]
- Goldin-Lang P, Tran QV, Fichtner I, et al. Tissue factor expression pattern in human nonsmall cell lung cancer tissues indicate increased blood thrombogenicity and tumor metastasis. Oncol Rep 2008;20:123-8. [PubMed]
- Streiff MB, Holmstrom B, et al. NCCN Guidelines Insights: Cancer-Associated Venous Thromboembolic Disease, Version 2.2018. J Natl Compr Canc Netw 2018;16:1289-303. [Crossref] [PubMed]
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018;36:2017-23. [Crossref] [PubMed]
- Khorana AA, Vadhan-Raj S, Kuderer NM, et al. Rivaroxaban for Preventing Venous Thromboembolism in High-Risk Ambulatory Patients with Cancer: Rationale and Design of the CASSINI Trial. Rationale and Design of the CASSINI Trial. Thromb Haemost 2017;117:2135-45. [Crossref] [PubMed]
- Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood 2007;110:1723-9. [Crossref] [PubMed]
- Pineo GF, Brain MC, Gallus AS, et al. Tumors, mucus production, and hypercoagulability. Ann N Y Acad Sci 1974;230:262-70. [Crossref] [PubMed]
- Sack GH, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore) 1977;56:1-37. [Crossref] [PubMed]
- Shao B, Wahrenbrock MG, Yao L, et al. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood 2011;118:4015-23. [Crossref] [PubMed]
- King MM, Nelson DA. Hypertrophic osteoarthropathy effectively treated with zoledronic acid. Clin Lung Cancer 2008;9:179-82. [Crossref] [PubMed]
- Martínez-Lavín M, Matucci-Cerinic M, Jajic I, et al. Hypertrophic osteoarthropathy: consensus on its definition, classification, assessment and diagnostic criteria. J Rheumatol 1993;20:1386-7. [PubMed]
- Yao Q, Altman RD, Brahn E. Periostitis and hypertrophic pulmonary osteoarthropathy: report of 2 cases and review of the literature. Semin Arthritis Rheum 2009;38:458-66. [Crossref] [PubMed]
- Ito T, Goto K, Yoh K, et al. Hypertrophic pulmonary osteoarthropathy as a paraneoplastic manifestation of lung cancer. J Thorac Oncol 2010;5:976-80. [Crossref] [PubMed]
- Davis MC, Sherry V. Hypertrophic osteoarthropathy as a clinical manifestation of lung cancer. Clin J Oncol Nurs 2011;15:561-3. [Crossref] [PubMed]
- Silveira LH, Martinez-Lavin M, Pineda C, et al. Vascular endothelial growth factor and hypertrophic osteoarthropathy. Clin Exp Rheumatol 2000;18:57-62. [PubMed]
- Hayashi M, Sekikawa A, Saijo A, et al. Successful treatment of hypertrophic osteoarthropathy by gefitinib in a case with lung adenocarcinoma. Anticancer Res 2005;25:2435-8. [PubMed]
- Santini D, Vincenzi B, Avvisati G, et al. Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin Cancer Res 2002;8:1080-4. [PubMed]
- Angel-Moreno Maroto A, Martínez-Quintana E, Suárez-Castellano L, et al. Painful hypertrophic steoarthropathy successfully treated with octreotide. The pathogenetic role of vascular endothelial growth factor (VEGF). Rheumatology (Oxford) 2005;44:1326-7. [Crossref] [PubMed]
- Bohan A, Peter JB. Polymyositis and dermatomyositis. II. N Engl J Med 1975;292:403-7. [Crossref] [PubMed]
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther 2010;12:R70. [Crossref] [PubMed]
- Zhang W, Jiang SP, Huang L. Dermatomyositis and malignancy: a retrospective study of 115 cases. Eur Rev Med Pharmacol Sci 2009;13:77-80. [PubMed]
- Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep 2011;13:208-15. [Crossref] [PubMed]
- Na SJ, Kim SM, Sunwoo IN, et al. Clinical characteristics and outcomes of juvenile and adult dermatomyositis. J Korean Med Sci 2009;24:715-21. [Crossref] [PubMed]
- Fujita J, Tokuda M, Bandoh S, et al. Primary lung cancer associated with polymyositis/dermatomyositis, with a review of the literature. Rheumatol Int 2001;20:81-4. [Crossref] [PubMed]
- Mori H, Habe K, Hakamada A, et al. Relapse of dermatomyositis after 10 years in remission following curative surgical treatment of lung cancer. J Dermatol 2005;32:290-4. [Crossref] [PubMed]
- Mukherjee S, Pandit S, Deb J, et al. A case of squamous cell carcinoma of lung presenting with paraneoplastic type of acanthosis nigricans. Lung India 2011;28:62-4. [Crossref] [PubMed]
- Kannenberg SM, Koegelenberg CF, Jordaan HF, et al. A patient with leonine facies and occult lung disease. Respiration 2010;79:250-4. [Crossref] [PubMed]
- Cohen PR, Grossman ME, Silvers DN, et al. Tripe palms and cancer. Clin Dermatol 1993;11:165-73. [Crossref] [PubMed]
- Yeh JS, Munn SE, Plunkett TA, et al. Coexistence of acanthosis nigricans and the sign of Leser-Trélat in a patient with gastric adenocarcinoma: a case report and literature review. J Am Acad Dermatol 2000;42:357-62. [Crossref] [PubMed]


