
Prevention of cognitive decline in old age—varying effects of interventions in different populations
Along with global population aging, dementia has posed tremendous challenges to the sustainable development of worldwide economy and society. In the past 3–4 decades, global extensive efforts have been made to develop new drugs for Alzheimer’s disease and dementia. However, effective curative treatments or even disease-modifying therapies are still not available for the devastating neurodegenerative disease (1). In the recent randomized clinical trial, Rovner and colleagues showed evidence that behavioural activation might prevent cognitive and functional decline among older black individuals with mild cognitive impairment (MCI) (2). In this commentary and mini-review, we highlighted the global burden of dementia and briefly summarized the evidence supporting the potential of non-pharmacological interventions to reduce the risk of cognitive disorders in old age, while a special attention was given to ethnic or racial variations.
Worldwide economic and societal burden of dementia
Cognitive function deteriorates as people age, which may represent normal part of age-related cognitive decline. However, genetic susceptibility (e.g., APOE genotype) and environmental factors (e.g., life-course unhealthy lifestyles, cardiometabolic risk factors, psychosocial factors, and chronic health conditions) may accelerate the pace of cognitive decline with age, and promote progression from the normal age-related cognitive decline to MCI, and further, to dementia. Indeed, dementia has posed tremendous challenges to our aging society. Worldwide, dementia affected ∼50 million people, and the number is projected to reach ∼75 million by 2030. The global cost of dementia was estimated at ∼US$1 trillion in 2018, and the total cost will reach US$2 trillion by 2030, driven primarily by accelerated global population aging. Thus, dementia has been recognized as a global public health priority by the World Health Organization (WHO) and Alzheimer’s Disease International (3,4) as well as by the London G8 Dementia Summit (www.gov.uk/government/news/uk-to-host-g8-dementia-summit). In China, nearly 10 million people are living with dementia. A conservative estimate of monetary costs of dementia in China was ∼US$70 billion (5), and the costs will increase dramatically over the coming decades (6). Currently, neither a cure nor a disease-modifying pharmacological therapy is available for dementia. Thus, identifying major risk and protective factors for accelerated cognitive decline and dementia, and further developing non-pharmacological interventions by targeting those modifiable factors to slow cognitive decline with age and delay the onset of clinical dementia have become an alternative strategy. Indeed, recent studies have shown a declining trend in incidence of dementia in some high-income countries (7,8), suggesting that late-life risk of dementia appears to be modifiable. However, there is evidence that occurrence (i.e., prevalence and incidence) of dementia, time trends of dementia occurrence, and risk and protective factors for dementia may vary across global populations with various ethnic and socio-cultural backgrounds (9). This suggests that one intervention approach does not fit all. The better or more effective interventions can be greatly facilitated through studies across different populations.
Time trends of dementia occurrence: regional and racial variations
In the past decade, numerous population-based studies have examined the time trends in prevalence and incidence of dementia, although the majority of the studies have been conducted in Europe and North America (1,7,8). Studies in Europe and North America have shown rather consistent evidence that since the 1980s, the age-specific prevalence of dementia has been relatively stable or slightly decreased, whereas the age-specific incidence of dementia might have decreased (7). By contrast, a limited number of studies in Asian populations (e.g., China and Japan) have shown an increase in both prevalence and incidence of dementia since the 1980s (10,11), although methodological issues may partly contribute to the increasing prevalence of dementia in China (7). In addition, evidence from studies of multi-ethnic populations has shown racial differences in the time trends of dementia occurrence such that dementia incidence has declined in whites and African-Americans but not African populations (12,13). The geographical and racial variations in the time trends of dementia occurrence have been supported by systematic reviews (7,14).
The major modifiable factors contributing to the regional or racial variations in the time trends of dementia occurrence are not fully understood. Methodological variations over time cannot fully explain the time trends. It has been hypothesized that the decline in incidence of dementia in Europe and North America might be partly attributable to the improvement in education and control of major cardiovascular risk factors (e.g., hypertension, diabetes, and high cholesterol or dyslipidemia) over time. Indeed, the increased educational attainment might increase the capacity of cognitive reserve possibly through positive effects on early-life brain development and lifespan mental stimulation and activities (e.g., cognitively-demanding jobs and leisure activities), thus, helping maintain late-life cognitive function. In addition, brain-healthy lifestyles (e.g., no smoking and regular physical exercise) and improved control of major cardiovascular risk factors may help maintain cardiovascular health and reduce the burden of cerebral macro- and microvascular lesions and neurodegenerative pathologies, which may contribute to the delayed onset of clinical dementia. This is partly supported by several well-designed studies showing that the late-life declining incidence of dementia or improvement in cognitive function over time may partly reflect a successful compression of cognitive morbidity along with increased life expectancy and longevity (8,15,16). In Asian countries, the upward trends in the age-specific prevalence and incidence of dementia since the 1990s in China are consistent with the increasing epidemic of unhealthy lifestyle and metabolic risk factors (e.g., physical inactivity, obesity, and diabetes) and related cardiovascular disorders (e.g., stroke and ischemic heart disease) during the similar time periods (8,10) as well as poor control of these major risk factors (17-19). In Japan, the increasing incidence of dementia is likely to be associated with a decrease in the competing risk of premature death and an increase in epidemic of unhealthy diet, physical inactivity, and diabetes (11). Although more research is needed to fully understand those factors contributing to the different time trends across geographic regions or races, the declining incidence of dementia in Europe and North America suggests that late-life risk of MCI and dementia seems modifiable, which provides the potential for non-pharmacological interventions to delay the onset of the dementia syndrome.
Non-pharmacological intervention studies in different populations
Older adults with MCI, especially amnestic MCI, are at a substantial risk for progression to dementia and Alzheimer’s disease. It has been hypothesized that the interventions will be more effective if implemented in the earlier phase of the disease process. The majority of small-scale intervention studies in patients with MCI have targeted a single domain such as cognitive intervention or physical exercise training, and the cognitive benefits of the single-domain interventions in MCI seem to be supported by systematic reviews and meta-analysis (20,21). Systematic reviews also concluded that multidomain interventions that combined both physical and cognitive components appeared to be more effective than the single-domain interventions in patients with MCI (20). There is limited evidence that lifestyle-based interventions may help maintain or improve cognitive function in Chinese older adults with MCI (22,23). Notably, numerous studies have tested whether multidomain lifestyle and behavioral interventions may benefit cognitive function in people with MCI, but few have focused on minorities such as black older adults. The 2-year randomized clinical trial that included 221 black individuals with amnestic MCI, showed evidence that the behavioral activation program, which was designed to increase cognitive, physical, and social activity (multidomain), driven by goal setting and action plans, could reduce the risk of cognitive decline compared with supportive therapy (a structured, nondirective psychological treatment that aimed to control for non-specific effect of social interactions) (2). The data showed that the effect size (differences in proportion of cognitive decline between the two groups) was increased with time. In this study, cognitive function was assessed with the Hopkins Verbal Learning Test-Revised (HVLT-R), and the predefined primary outcome was a decline of ≥6 recalled words on the HVLT-R test at 24 months. Of note, the study participants (age ≥65 years, mean 75.8 years; 79.2% women; and mean educational level 12.5 years) were derived from diverse settings (e.g., senior centers, nursing homes, churches, and primary care clinics). The biological and neuropathological pathways of the cognitive benefits of the behavioral activation program are not fully understood, although the multidomain interventions could possibly contribute to both reduced cardiovascular lesions and increased capacity of cognitive reserve. In addition, evidence has indicated racial disparities in global cognitive decline and dementia risk, e.g., African-Americans often experience more cognitive decline or a double-risk of dementia than Caucasians; the rate of cognitive decline and risk of dementia in minorities (e.g., African-Americans) could potentially be modified by educational attainments and health literacy (24,25). Therefore, the clinical relevance and generalizability (e.g., with regard to ethnic background, socioeconomic status, and health literacy) of the findings and the underlying neuroprotective mechanisms deserve further investigation.
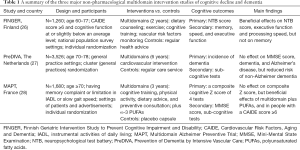
In addition, given the multifactorial nature of the accelerated cognitive decline with age and dementia, simultaneously targeting multiple risk and protective factors might be essential for any interventions to be effective. Several lifestyle-based multidomain intervention studies have been completed in European populations (26-28) (Table 1). The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) is a landmark non-pharmacological intervention study of this kind, which showed that a multidomain lifestyle intervention, based on simultaneous management of several vascular and lifestyle-related risk factors for dementia, can benefit cognitive function in older adults (age 60–77 years) at elevated risk of dementia (26). In addition, the Dutch Prevention of Dementia by Intensive Vascular Care (PreDIVA) trial sought to test the efficacy of a 6-year, nurse-led, multidomain cardiovascular intervention (vs. usual care) for the prevention of dementia. The trial did not show an overall effect on the risk of dementia (primary outcome), but did show a protective effect for non-Alzheimer dementia (27). Finally, the French Multidomain Alzheimer Preventive Trial (MAPT) tested the efficacy of a multidomain lifestyle intervention program (e.g., cognitive training, dietary advice, and physical activity) alone or in combination with n-3 polyunsaturated fatty acid supplementation, on cognitive decline among frail individuals aged ≥70 years. This study failed to demonstrate the overall effect of the intervention program on the risk of dementia (primary outcome), but the multidomain intervention did benefit cognitive function for individuals at increased risk of dementia (28).
Full table
The World Wide FINGERS Initiative represents a global coordinated effort that seeks to test the cognitive benefits of the FINGER’s intervention model in different elderly populations and socio-cultural settings across the world (www.alz.org/wwfingers/overview.asp). The ongoing multimodal interventions to delay the onset of dementia and disability in rural China (MIND-CHINA) study will test whether a program of improving control of multiple cardiovascular risk factors (i.e., high blood pressure, high blood glucose, and high cholesterol) and a multimodal intervention program that integrates improvement in control of major vascular risk factors with multiple cognitive stimulating activities will help maintain cognitive and physical function among rural-dwelling older adults (age 60–79 years) in Shandong province, China. In addition, the US study to protect brain health through lifestyle intervention to reduce risk (US-POINTER) is designed as a 2-year trial that aims to test the multidomain intervention in adults aged 60–79 years who are at high risk for cognitive decline. Similar multidomain intervention studies are being planned in countries such as Singapore (SINGER), the UK (UK-FINGER), Canada, Germany, Japan, and Spain. Given that data are currently lacking with regard to cognitive benefits of multidomain interventions among Asian populations and that both prevalence and incidence of dementia have increased in Asian countries, it is highly relevant that the World Wide FINGER initiative is joined by Asian countries.
Potential different effects of interventions among different ethnic populations
Studies of multi-ethnic populations have shown evidence for racial disparities of cognitive decline and dementia risk, such that African-Americans have the highest incidence of dementia, followed by Latinos and whites, and Asian populations have the lowest risk of dementia (29). Research has suggested that the greater risk of dementia in blacks, as compared with whites, may be partly due to poorer cognitive performance (at baseline) in blacks than whites, possibly resulting from lower educational attainment, lower socioeconomic status, and higher prevalence of vascular risk factors and related disorders in blacks (25). This has implication for designing intervention studies to test the racial differences in the effect of interventions. Indeed, there is evidence that disparities in dementia occurrence between whites and blacks have declined in the past decades, driven by larger decline in prevalence of dementia in blacks, whereas disparities between whites and Hispanics increased over this time period (30). Theoretically, multidomain intervention programs that are aimed at reducing the risk of cardiovascular events or at improving the management of major cardiovascular risk factors and related vascular diseases may also benefit cognitive function and lower the risk or delay the onset of dementia (31). These intervention programs should consider the complex combination of socioeconomic and socio-cultural factors as well as risk and protective factors that are associated with racial or ethnic disparities in dementia risk. In particular, future research should pay more attention to identifying preventive and therapeutic intervention options for racial and ethnic minorities by recruiting diverse participants into the intervention studies.
Conclusions and future perspectives
In the past three decades, the better understanding of the modifiable risk and protective factors for cognitive decline and dementia from the life-course perspective represents one of the major progresses in research of epidemiology of dementia and other cognitive disorders in aging. However, intervention studies are urgently needed to translate the knowledge from observational studies into benefits of healthy aging overall, and healthy brain aging in particular, of the public. Recent intervention studies have shown evidence that non-pharmacological multidomain interventions may help maintain cognitive function in older adults at risk for cognitive aging, but further research is needed to provide more convincing evidence for the cognitive benefits. In particular, gender or sex differences in risk and protective factors for cognitive aging and dementia have been well recognized (32), future studies should pay more attention to racial or ethnic differences in the influential factors of MCI and dementia. This may help achieve precision preventive medicine for the intervention of cognitive decline and dementia. In this context, future intervention studies should take into account diversities of the populations in terms of demographic features (e.g., sex, race, and socioeconomic position) and socio-cultures. The ongoing World Wide FINGERS Initiative may help achieve the goals.
Acknowledgments
Funding: B Winblad and F Zhu received grants from “The Three Famous Project” for the Shenzhen-Luohu Hospital – Karolinska Institutet collaboration. B Winblad received grants from the Swedish Research Council and from the Margaretha af Ugglas Foundation. C Qiu received grants from the Taishan Scholar Program of Shandong Province, Shandong, China, the Swedish Research Council for Health, Working Life and Welfare, and the Swedish Research Council for Sweden-China Collaboration (2017-00740, and 2017-05819), Sweden. M Kivipelto received grants from the Swedish Research Council, Alzheimerfonden, Stockholm County Council, AXA Research Fund, Knut and Alice Wallenberg Foundation, Center for Innovative Medicine at Karolinska Institutet Sweden; Stiftelsen Stockholms Sjukhem, and Konung Gustaf V:s och Drottning Victorias Frimurarstiftelse, Sweden.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol 2016;15:455-32. [Crossref] [PubMed]
- Rovner BW, Casten RJ, Hegel MT, et al. Preventing cognitive decline in Black individuals with mild cognitive impairment: A randomized clinical trial. JAMA Neurol 2018;75:1487-93. [Crossref] [PubMed]
- World Health Organization (WHO) and Alzheimer’s Disease International. Dementia: a public health priority. Geneva: WHO, 2012.
- Wimo A, Guerchet M, Ali GC, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 2017;13:1-7. [Crossref] [PubMed]
- Xu J, Wang J, Wimo A, et al. The economic burden of dementia in China, 1990-2030: implications for health policy. Bull World Health Organ 2017;95:18-26. [Crossref] [PubMed]
- Jia J, Wei C, Chen S, et al. The cost of Alzheimer's disease in China and re-estimation of costs worldwide. Alzheimers Dement 2018;14:483-91. [Crossref] [PubMed]
- Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol 2017;13:327-39. [Crossref] [PubMed]
- Qiu C, Fratiglioni L. Aging without Dementia is Achievable: Current Evidence from Epidemiological Research. J Alzheimers Dis 2018;62:933-42. [Crossref] [PubMed]
- Alladi S, Hachinski V. World dementia: One approach does not fit all. Neurology 2018;91:264-70. [Crossref] [PubMed]
- Chan KY, Wang W, Wu JJ, et al. Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet 2013;381:2016-23. [Crossref] [PubMed]
- Ohara T, Hata J, Yoshida D, et al. Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology 2017;88:1925-32. [Crossref] [PubMed]
- Gao S, Ogunniyi A, Hall KS, et al. Dementia incidence declined in African-Americans but not in Yoruba. Alzheimers Dement 2016;12:244-51. [Crossref] [PubMed]
- Noble JM, Schupf N, Manly JJ, et al. Secular trends in the incidence of dementia in a multi-ethnic community. J Alzheimers Dis 2017;60:1065-75. [Crossref] [PubMed]
- Stephan BCM, Birdi R, Tang EYH, et al. Secular Trends in Dementia Prevalence and Incidence Worldwide: A Systematic Review. J Alzheimers Dis 2018;66:653-80. [Crossref] [PubMed]
- Jagger C, Matthews FE, Wohland P, et al. A comparison of health expectancies over two decades in England: results of the Cognitive Function and Ageing Study I and II. Lancet 2016;387:779-86. [Crossref] [PubMed]
- Leggett A, Clarke P, Zivin K, et al. Recent improvements in cognitive functioning among older U.S. adults: How much does increasing educational attainment explain? J Gerontol B Psychol Sci Soc Sci 2019;74:536-45. [Crossref] [PubMed]
- Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948-59. [Crossref] [PubMed]
- Song A, Liang Y, Yan Z, et al. Highly prevalent and poorly controlled cardiovascular risk factors among Chinese elderly people living in the rural community. Eur J Prev Cardiol 2014;21:1267-74. [Crossref] [PubMed]
- Lu J, Lu Y, Wang X, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1·7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet 2017;390:2549-58. [Crossref] [PubMed]
- Sherman DS, Mauser J, Nuno M, Sherzai D. The efficacy of cognitive intervention in mild cognitive impairment (MCI): a meta-analysis of outcomes on neuropsychological measures. Neuropsychol Rev 2017;27:440-84. [Crossref] [PubMed]
- Karssemeijer EGA, Aaronson JA, Bossers WJ, et al. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res Rev 2017;40:75-83. [Crossref] [PubMed]
- Lam LC, Chan WC, Leung T, et al. Would older adults with mild cognitive impairment adhere to and benefit from a structured lifestyle activity intervention to enhance cognition?: a cluster randomized controlled trial. PLoS One 2015;10:e0118173. [Crossref] [PubMed]
- Li M, Liu L, Song S, et al. Effect of long-term lifestyle intervention on mild cognitive impairment in hypertensive occupational population in China. Medicine (Baltimore) 2018;97:e11975. [Crossref] [PubMed]
- Gupta VK, Winter M, Cabral H, et al. Disparities in age-associated cognitive decline between African-American and Caucasian populations: The roles of health literacy and education. J Am Geriatr Soc 2016;64:1716-23. [Crossref] [PubMed]
- Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive aging in Black and White Americans: Cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology 2018;29:151-9. [Crossref] [PubMed]
- Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomized controlled trial. Lancet 2015;385:2255-63. [Crossref] [PubMed]
- Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 2016;388:797-805. [Crossref] [PubMed]
- Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol 2017;16:377-89. [Crossref] [PubMed]
- Mayeda ER, Glymour MM, Quesenbrry CP, et al. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016;12:216-24. [Crossref] [PubMed]
- Chen C, Zissimopoulos JM. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement (N Y) 2018;4:510-20. [Crossref] [PubMed]
- Qiu C, Fratiglioni L. A major role of cardiovascular burden in cognitive decline. Nat Rev Cardiol 2015;12:267-77. [Crossref] [PubMed]
- Ferretti MT, Iulita MF, Cavedo E, et al. Sex differences in Alzheimer disease - the gateway to precision medicine. Nat Rev Neurol 2018;14:457-69. [Crossref] [PubMed]


