
Pompe disease gene therapy: neural manifestations require consideration of CNS directed therapy
Introduction
Gene replacement strategies are best suited to single gene defects where augmentation of expression or complete restoration of wild type activity levels is required to impact the clinical phenotype. Of course, even achieving the heterozygous level of gene expression should have a therapeutic effect in Pompe disease since carriers are entirely asymptomatic. Directing a gene therapy vector to the primarily affected systems can be influenced by route of delivery; however, with the advent of systemic vector delivery, tissue restricted expression is mostly achieved through promoter selection. Early treatment would have the benefit of preventing secondary manifestation of the disease. In addition, when progressive cellular dysfunction leads to cell death, early intervention is warranted. In this review we present evidence of the extensive neuropathology in Pompe disease in association with the metabolic myopathy classically considered the primary pathology. The neuropathology observed in three animal models of Pompe and in human autopsy studies are a key justification for gene therapy with the overarching goal of direct cell autonomous correction in the brain and spinal cord. In non-clinical studies, we have demonstrated that pathophysiology is directly related to GAA gene function and restoration of GAA activity leads to reversal of glycogen accumulation and improved cellular function. Another important consideration in recessive conditions and Pompe in particular is the impact of immune response to the transgene when there is an effective null phenotype. Such cases require both tissue restricted expression and strategies to induce immune tolerance to the transgene. Lastly, as a lysosomal protein, GAA selectively traffics to the lysosome and achieving cell autonomous correction takes advantage of the highly efficient system of endo-lysosomal transport rather than relying on relatively inefficient binding of the therapeutic protein to the extracellular mannose-6-phosphate receptor for internalization.
Because of the cardiac and skeletal muscle involvement in Pompe disease, the condition is often considered a metabolic myopathy and a member of the general category of muscular dystrophy. Acid alpha-glucosidase is however required for degradation of lysosomal glycogen in all tissues and therefore manifestations are systemic. One explanation for the differential manifestations across tissue beds is that the disease severity in an organ system may be related as much to the cellular program for glycogen synthesis in each tissue as to the rate of glycogen degradation. This consideration is supported by cases where a second mutation leading to increased glycogen synthesis is especially severe when there is hemizygous expression of GAA [Austin et al. (1) and Shebab in preparation]. Currently over 10 years of enzyme replacement therapy (ERT) experience has been established in Pompe disease (2-4) and children and adults on ERT now demonstrate a modified natural history compared to untreated historical controls. The findings from longitudinal registry studies will be important to continue to understand the strengths and weakness of individual therapies that have achieved marketing approval (5,6). Many observational studies in the treated patient population are defining the benefits and key limitations of ERT, especially related to the neurological manifestation of the disease (7-11).
Our group has emphasized the importance of establishing meaningful preclinical models to establish a better understanding of the pathophysiology in Pompe disease and allow for the most impactful human clinical studies for gene therapy (12-17). One goal of this review is to present the basis for gene therapy in Pompe disease by consideration of the non-clinical proof of concept data predominantly derived from the Pompe mouse model (18). The null (Gaa−/−) mouse has been a useful model and matched the biochemical phenotype of infantile onset Pompe disease (IOPD). However, the lack of early mortality in the Gaa−/− mouse has led us to develop new murine models as well as rat and canine Pompe models to better enable adequately planning for clinical studies in both early- and late-onset disease.
In vitro studies supporting biochemical correction and cross-correction
Pompe patient derived cells were used initially to test the concept that gene augmentation would lead to sufficient expression of human GAA and that the vector-derived protein would reach the lysosome (19-21). Cross-correction was also demonstrated in these studies using a trans-well system. The same studies established the basis for ERT. New cell culture systems have also emerged as a way to model Pompe disease by induced pluripotent stem cells (iPSC) or specific cell types derived from iPSC (22-26). Targeting the murine GAA allele has produced two strains of mice which vary in clinical phenotype (18,27). In addition, differing genetic backgrounds has helped to emphasize the importance of modifier genes on disease severity.
Beyond the mouse model, we have now developed and characterized a knockout rat model of Pompe disease. The rat was created using a zinc finger nuclease (ZFN) to create a ten base pair deletion in the Gaa gene resulting in a global GAA knockout (Falk, in preparation, 2019). In this rat model, the observed cardiomyopathy is more representative of the human disease. In fact, the average age of death is 7.3 months in female rats and 4.9 months in male rats (Falk, in preparation, 2019).
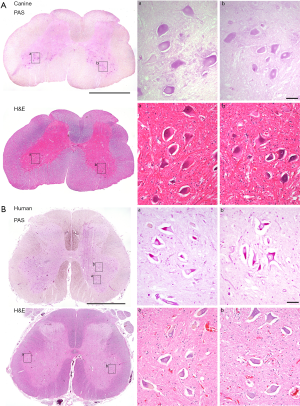
The final model we have generated to facilitate the non-clinical studies supporting Pompe disease gene therapy is the canine model. Seppälä et al. (28) characterized the genealogy of a naturally occurring nonsense codon mutation in exon 16 of the canine Gaa locus (c.2237G>A; W746*). Carrier females were generated by crossing a purebred Finnish Laphund carrier male with a female black Labrador. Natural history data of clinical cases confirmed the rapid onset of cardiomyopathy and early mortality in affected animals. Importantly, lumbar spinal cord histology of untreated pups indicates pathology and glycogen accumulation in motoneurons (Figure 1), suggesting this model is representative of Pompe disease in humans. Treatment with systemic gene transfer is underway to evaluate longevity of expression. Initial findings show that rapid progression of motoneuron pathology is not reversible with AAV-mediated gene transfer when therapy is initiated after 6 months of age in the dog, which corresponds to 5–10 years of age in human (personal communication Byrne). Conversely, early treatment and immune management promote normal muscle development and strength. Further studies to establish the optimal regimen for immune tolerance induction are in progress.
Cardiac gene therapy in Pompe disease
In the pivotal studies to evaluate Myozyme and subsequently Lumizyme, all early-onset patients with severe missense mutations or nonsense mutations had severe and progressive cardiomyopathy leading to early mortality (29-34). Cardiorespiratory failure at an average age of eight months of age is the principle cause of death in the natural history study done in preparation for the first ERT treatment study (1). Evidence of early effects on cardiomyopathy are likely the primary reason for improved survival (31,35) and therefore a gene therapy strategy in IOPD must consider and specifically target myocardium. Also notable is that those with the most severe mutations have an incomplete response to ERT and increase in LV mass is observed in the face of anti-drug antibodies (36). A gene therapy strategy can result in direct cell autonomous correction of myocardium in non-clinical studies therefore leading to reduction in LV mass index and improved cardiac function.
The Gaa−/− mouse model (18) has been critical in developing new therapeutic strategies and understanding the pathophysiology in Pompe disease. Importantly, the model has also been the principal test bed for progress in gene therapy. The model has features of both early and late onset disease and lives a full life-span since the cardiac manifestations are less severe than in humans with a null mutation. Structural and functional abnormalities in the heart are apparent in Gaa−/− mice by 6 months of age by examining LV mass index and the electrocardiogram (18,37-39). Difficulty in rearing and feeding lead to weight loss after 18 months of age and are sometimes the cause for euthanasia.
We have demonstrated that high-field cardiac magnetic resonance imaging (MRI) can be used to monitor for left ventricular hypertrophy and impaired ejection fraction in Gaa−/− mice (15,16,37,40,41). We and others have established the basis for AAV-mediated expression of hGAA in cardiac tissue and clearance of glycogen following systemic AAV dosing or via cross-correction from liver-mediated production of GAA (15-17,21,37,40-47) (Mc Call, 2019, submitted). Importantly, the exposure of AAV9 to the cardiac musculature is far more efficient than in skeletal muscle. Indeed, a single intravenous adeno-associated virus (AAV) vector injection results in extensive transduction and high-level hGAA expression. The high level expression leads to restoration of cardiac function at all stages of disease progression (37,40,44,48,49). Systemic administration of AAV1-CMV-hGAA results in ~70% reduction in cardiac lysosomal glycogen one-year post-dosing (37). Other outcomes include correction of the common electrophysiological abnormality and increased LV mass (15,37). Beyond the experience with AAV1, we found that the strong tropism of AAV9 for myocardium and the brain make this a suitable candidate for human studies. For example, the level of gene expression following systemic delivery of AAV9-CMV-LacZ resulted in a 200 fold increase in expression versus the identical vector DNA packaged into AAV1 (41). The systemic administration of a an rAAV2/9 GAA vector with a modified promoter to restrict expression to muscle and neural cells has been subsequently used in a number of mouse, rat and canine studies (40,44). Even in the setting of existing cardiac and skeletal muscle abnormalities, treatment of newborn or adult Gaa−/− mice can result in physiological and electrical correction of cardiac and skeletal muscles. Quantitative glycogen measurement, PAS staining, and electron microscopy are the most favorable methods to demonstrate clearance of glycogen and correction of sub-cellular morphology (40). Studies in rhesus macaques were also conducted to confirm the novel finding of significantly increased activity in heart (5× over baseline after 6 months) (41). Additional evaluation of the safety and efficacy of AAV-GAA in primates have been complete with support of the NHLBI Gene Therapy Resource program (50). These studies confirm the initial observations with systemic delivery of AAV-GAA and go on to establish the basis for immune protection versus the AAV capsid and transgene in Pompe gene therapy (51).
The set of studies described above demonstrate the efficacy of systemically delivered AAV-GAA and confirm extensive transduction in the heart and subsequent reduction of glycogen content with reversal of cardiac hypertrophy and dysfunction. Ongoing studies in the rat and canine models will determine the potential for benefit in the setting of severe cardiac hypertrophy as well as durability following systemic delivery.
AAV gene therapy for the muscle phenotype in Pompe disease
Since the initial description of the Pompe null mouse model (18) we have generated several derivative strains to better understand aspects of disease pathology. To study immune management, we generated a mouse line expressing human CD20 (52) by crossing human CD20 transgenic mice into the null GAA background. The resulting mice allow for testing anti-B cell strategies based on human CD20. Studies with this line have elucidated both the effect of prolonged administration of ERT (51,53,54) (a novel antigen in the KO mice) and for the administration of gene therapy vectors expressing the human gene (50,55). Additionally, mouse lines have been generated with deletion of floxed exon 2 (unpublished), ectopic expression of GAA (56) and a known human missense (54,57) mutation to evaluate the findings of gene transfer in the setting where the human vector derived protein is considered a self-protein.
We utilized isometric force-frequency relationships from diaphragm muscle and soleus muscle from the Gaa−/− line to demonstrate a progressive decline in contractile strength with age (58). The Pompe mice also show reduced ventilatory function as seen in juvenile and adult-onset patients (13). Given these baseline observations, we sought to show that systemic delivery of the AAV-GAA vector would have the maximal benefit in the context of gene therapy. It is important to emphasize that subcellular trafficking of lysosomal proteins has evolved a specific mechanism for retention of the lysosomal proteins to facilitate targeting to the lysosome. The strategy of cell autonomous correction should be highlighted as fundamentally different from liver-mediated cross correction (59). Specifically, the efficiency of cross-correction is influenced by the mannose-6-phosephate receptor density in the peripheral musculature and, therefore, is several logs less efficient than the intracellular trafficking of GAA since transit from trans-Golgi to the lysosome is the pathway which has evolved to capture bis-mannose-6-phosphate lysosomal proteins.
Mah et al. (37) showed that systemic delivery of rAAV2/1 to Gaa−/− neonatal mice led to correction of GAA enzymatic activity and glycogen clearance in striated muscle for up to one year. In addition, there was evidence of physiological correction by measurement of soleus and diaphragm force mechanics to 90% of wild-type peak diaphragm contractile strength and concomitant increase in ventilatory function. Adult Gaa−/− mice showed significantly reduced glycogen content in striated muscle and diaphragm at 4.5 months post-dosing with either rAAV2/8 or rAAV2/9 vectors where expression is restricted to striated muscle (44). Additional evidence of skeletal correction is derived from ex vivo force mechanics on excised Gaa−/− diaphragms following systemic rAAV2/9 GAA vector as well as X-ray analysis demonstrating a decrease in spinal scoliosis and kyphosis (40). The strong affinity of rAAV2/9 vectors for a variety of muscle fiber types as well as neurons and cardiac muscle is advantageous compared to other rAAV serotype vectors as a therapeutic vector for muscular dystrophies (41,44,48). Liver-directed delivery of rAAV2/5 and rAAV2/8 vectors using a cross-correction strategy can increase enzyme levels in the diaphragm and hind-limb muscles with concomitant reduction of glycogen content (17,42,59). Importantly, Puzzo et al. found 50% reduction in CNS glycogen content after one year of therapy (59) . The mechanism of glycogen reduction is presumably via hematopoietic cells that carry GAA across the blood-brain barrier, but it is unclear if this will be sufficient to provide clinical benefit in humans. To obtain a more direct effect, the same group has proposed an hybrid promoter which is active in muscle and the liver or CNS and the liver (60). Additional considerations for in vivo non-clinical studies include the age of the animal, species-specific specificity of the AAV receptor and the degree of expression of mannose-6-phosphate receptor in the affected tissues (46,47,49,58).
The only commercially approved therapy for Pompe disease is alglucosidase alfa, Lumizyme® (US) and Myozyme® (ex-US) which is a recombinant protein used for ERT. ERT improves ventilator-free survival rates in patients with infantile-onset disease, but longer-term follow-up showed a significant proportion showing progressive loss of ventilatory function (22 of the original 38 subjects now either require assisted ventilation or have died). All subjects have demonstrated functional deficits in respiratory function and some aspects of disease progression are not eliminated. Based on these findings we have intensified the effort to better understand the incomplete response to ERT to identify additional therapeutic strategies using gene therapy.
AAV vectors can be delivered either systemically or directly in regional dosing for proof of concept. Direct diaphragm delivery was tested to focus on the reversibility of phrenic motor and diaphragm contractile function (43,61). Targeted administration of rAAV2/1-CMV-hGAA to the diaphragm of Gaa−/− mice resulted in near wild-type levels of diaphragm GAA activity and the clearance of accumulated glycogen, both in younger animals and 2-year-old mice with established disease. All treated mice had improved diaphragm contractile function and in vivo ventilatory capabilities, however disease progression impacts the potential for improvement and is influenced by the degree of neuronal loss with advanced age (62,63). It will be necessary to test these therapeutic strategies in human subjects at varying stages of disease severity to determine the degree of reversibility in either muscle pathology or neuronal cell dysfunction (58,61,64).
Evidence for CNS manifestations of Pompe disease amenable to gene therapy
The degree of weakness in Pompe disease patients has historically been attributed predominantly to muscle pathology (65). Yet, there is long-standing evidence that glycogen accumulates in the central nervous system (CNS) of Pompe patients (13,66-70) and in animal models of the disease (13,62,63,71,72). Neurological motor symptoms have been reported in case studies (11,70,73-76) and one report describes possible cognitive deficiencies (77). Studies in the Gaa−/− mouse demonstrate glycogen accumulation in phrenic motoneurons and diminished phrenic motor output leads to impaired ventilation (13,58,78-83). We have reported autopsy findings of cellular pathology in spinal motoneurons of a Pompe infant treated with Myozyme (13) and we have recently had the opportunity to evaluate CNS pathology in an adult (age 55) who directed that upon his death, an autopsy would be performed to evaluate CNS pathology (personal communication). Figure 1 shows lumbar spinal cord sections from this individual who had been on long term ERT at the time of his death. The clear demonstration of motoneuron glycogen accumulation and histopathology are consistent with the observation that ERT does not effectively cross the blood-brain-barrier (39,84) and therefore cannot impact GAA deficiency in the CNS. It is possible that the variability in the success of enzyme replacement (85) could reflect persistent (untreated) CNS pathology. In fact, Muller et al. reported that children with Pompe disease remain at high risk for speech disorders despite ongoing ERT (86) and the same group has recently report neuroimaging findings in children with Pompe disease (87). Figure 1 also presents lumbar spinal sections from the canine Pompe model, with similar evidence of motoneuron histopathology.
The principal focus of our group has been to establish AAV-mediated therapies which target both cardiac and skeletal muscle as well as the CNS (7,13,15,37,64,78-80,88-90). We have evaluated CNS targeting by direct administration, retrograde transport of vector, or after systemic or sub-arachnoid delivery (91,92). The first observation of retrograde transport was following administration of rAAV2/1 to the diaphragm of an adult Gaa−/− mouse leading to increased efferent phrenic nerve activity (58). Subsequent studies have shown robust transduction of hypoglossal motoneurons following intra-lingual delivery or leg motoneurons following tibialis treatment (78-81,83,93). Todd et al. observed that older animals treated with AAV-GAA can have restoration of muscle GAA activity and glycogen reduction but electrical stimulation of the peroneal nerve is unable to generate force at the ankle (81,83). In contrast, neuromuscular junction abnormalities can be restored with AAV-GAA in younger animals, and the contrast in therapeutic efficacy between young and old mice likely reflects loss of lower motoneurons over time. Consistent with that hypothesis, transcriptome analysis of the Gaa−/− mouse spinal cord indicated activation of molecular pathways associated with cell death, and neuronal apoptosis was confirmed with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (63).
Immunological considerations for Pompe disease gene therapy
The current standard of care IOPD is ERT with alglucosidase alfa (Lumizyme®). In LOPD there is an active debate about when to initiate therapy. For example, is preventative treatment in asymptomatic patients warranted? In the era of newborn screening, this debate will continue as more data on pre-symptomatic patients are collected (94). Other supportive data from imaging (87,94-97) and blood biomarker studies (98) will be important in establishing a basis for treatment and by what approach.
In IOPD with severe missense or nonsense mutations, ERT has resulted in high sustained anti-GAA antibody titers in most patients and in turn, there is a high rate of infusion reactions (33,34,99). Similarly, Gaa−/− mice generate strong humoral immune responses to recombinant human GAA (53,54). Both IgG and IgE anti-drug antibodies have been observed, which likely causes the fatal anaphylactic reactions that occur in these animals after repeated ERT (100). While the acute response can be suppressed with anti-histamine drugs, the anti-drug antibodies are still able to bind the recombinant protein and cause aggregation which reduces efficacy.
It is possible to induce tolerance to GAA by pretreating with hepatic AAV-GAA gene transfer which prevents the predisposition to anaphylactic reactions (100). These observations are based on the pioneering work by Herzog et al., where hepatocyte-derived expression induces transgene product-specific immune tolerance (101-103) in which regulatory T-cells actively suppress B and T cells (102,104,105). Additional approaches toward immune tolerance induction have been developed using immune suppressive drugs such as rapamycin in combination with B cell-depleting antibodies (36,50,101).
The anti-transgene response is influenced by the underlying GAA mutation. An additional consideration is the anti-capsid response in which the innate and adaptive immune system responds to the novel vector capsid protein thereby clearing the vector from the circulation and limiting efficacy. There is some evidence that AAV can induce a transient innate response accompanied by complement activation, TLR-9 signaling, and plasmacytoid dendritic cell activation which can be significant at the higher doses used in systemic therapy (106). Another important aspect of anti-capsid immunity is an environmental exposure, which would prime the individual to a secondary or amnestic response if exposed to a therapeutic vector of the same or related serotype (107). Important variables to be considered in regards to adaptive responses to AAV include the vector serotype, route of administration, dose, promoter and the targeted organ (42,49,103,108,109). We have also considered the use of liver-directed gene therapy (AAV2/8-LSP-hGAA) followed by ERT to reduce IgE mediated hypersensitivity (42,51,100). Strategies to transiently suppress the immune response have been used to circumvent innate and adaptive responses in gene therapy and ERT for Pompe disease and other inherited protein deficiency disorders (101,110). We have begun two studies to evaluate transient immune suppression in the context of AAV-medicated gene therapy and the initial results demonstrate the potential to increase efficacy and enhance safety (9,50,55).
Human clinical experience with AAV-GAA therapy
In the current era of an approved ERT for Pompe disease and the implementation of newborn screening across the US and Taiwan, there is a need for adjunctive therapy which will address the remaining unmet need in the current Pompe population and those identified by newborn screening. Therefore, a successful gene therapy strategy for Pompe disease should impact the disease manifestations which are defined by the current natural history of both treated and newly diagnosed patients. The first gene therapy in Pompe disease was an open label, Phase I/II study administering rAAV2/1-CMV-hGAA by direct intramuscular injection to the diaphragm of children (age 3–14) with ventilatory failure despite ERT (ClinicalTrials.gov Identifier: NCT00976352) (9,111,112). That study confirmed safety and indicated benefit to respiratory function in some patients (113). An ongoing trial assesses the ability to re-administer an AAV9 vector intramuscularly in patients with late-onset Pompe disease (ClinicalTrials.gov Identifier: NCT02240407). The study is a blinded crossover design to test safety and effectiveness of administration and re-administration of AAV9-DES-hGAA vector injected intramuscularly into the tibialis anterior (TA) muscle. In this study, the immune modulation strategy is to transiently ablate B-cells (Rituximab) and modulate T-cell response (Sirolimus) prior (and after) the initial exposure to AAV9 in one leg and the subsequent exposure of the same vector to the contralateral leg after four months. Preliminary results in 2 subjects show that immunomodulation successfully prevents antibody formation against both the AAV capsids and the transgene, and allows for repeated exposure to the vector. In addition, immunomodulation during AAV administration increases the efficacy and duration of the treatment (Corti oral communications). Based on preclinical and clinical results supporting the immunomodulation approach, a new clinical trial in children with Pompe disease will be initiated at the NIH Clinical Center, Bethesda, MD. The study is a Phase I–II trial of systemic delivery of rAAV9-DES-GAA in children with IOPD (3–5 years old) in conjunction with immunomodulation (Rituximab and sirolimus).
Conclusions
Currently, there is substantial evidence of neuropathology in Pompe disease, which influences the efficacy of treatment approaches based on ERT and liver-mediated gene therapy strategies. Targeting of CNS and muscle using novel AAV serotypes will be critical for a successful therapy. Additionally, management of immune responses against the vector capsid and the transgene would be required to increase treatment safety and efficacy. The knowledge gained from many non-clinical studies and initial clinical trials is paving the way for a truly transformative therapy for the Pompe patient community.
Acknowledgments
The authors gratefully acknowledge the University of Florida Powell Gene Therapy Center Vector Core Laboratory for production of rAAV vectors for preclinical studies, the UF Toxicology Core Laboratory for vector toxicology analysis, and the Human Applications Laboratory, which produced the rAAV vectors for clinical studies. Special thanks to Denise Cloutier, Megan Pope and Marda Jorgensen for managing the preclinical studies and contributing to data analysis. Special thanks to Nina Raben for providing the original strain of Gaa−/− mice and organizing this special issue.
Funding: This work was partially supported by grants from the National Institutes of Health (NHLBI P01 HL59412-06, NIDDK P01 DK58327-03, 1R01HD052682-01A1, and the NHLBI Gene Therapy Resource Program. B.J.B., D.D.F.; The Johns Hopkins University, and the University of Florida could be entitled to patent royalties for inventions described in this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Austin SL, Chiou A, Sun B, et al. Alglucosidase alfa enzyme replacement therapy as a therapeutic approach for a patient presenting with a PRKAG2 mutation. Mol Genet Metab 2017;120:96-100. [Crossref] [PubMed]
- Kishnani PS, Hwu WL, Mandel H, et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 2006;148:671-6. [Crossref] [PubMed]
- Van der Beek NA, Hagemans ML, Reuser AJ, et al. Rate of disease progression during long-term follow-up of patients with late-onset Pompe disease. Neuromuscul Disord 2009;19:113-7. [Crossref] [PubMed]
- van der Meijden JC, Kruijshaar ME, Harlaar L, et al. Long-term follow-up of 17 patients with childhood Pompe disease treated with enzyme replacement therapy. J Inherit Metab Dis 2018;41:1205-14. [Crossref] [PubMed]
- Byrne BJ, Kishnani PS, Case LE, et al. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol Genet Metab 2011;103:1-11. [Crossref] [PubMed]
- Kishnania P, Chien YH, Llerena J, et al. The Pompe Registry: 10 Years of Data. J Neuromuscul Dis 2015;2:S22-3. [PubMed]
- Fuller DD, ElMallah MK, Smith BK, et al. The respiratory neuromuscular system in Pompe disease. Respir Physiol Neurobiol 2013;189:241-9. [Crossref] [PubMed]
- Hordeaux J, Dubreil L, Robveille C, et al. Long-term neurologic and cardiac correction by intrathecal gene therapy in Pompe disease. Acta Neuropathol Commun 2017;5:66. [Crossref] [PubMed]
- Corti M, Liberati C, Smith BK, et al. Safety of Intradiaphragmatic Delivery of Adeno-Associated Virus-Mediated Alpha-Glucosidase (rAAV1-CMV-hGAA) Gene Therapy in Children Affected by Pompe Disease. Hum Gene Ther Clin Dev 2017;28:208-18. [Crossref] [PubMed]
- Smith BK, Corti M, Martin AD, et al. Altered activation of the diaphragm in late-onset Pompe disease. Respir Physiol Neurobiol 2016;222:11-5. [Crossref] [PubMed]
- Corti M, Smith BK, Falk DJ, et al. Altered activation of the tibialis anterior in individuals with Pompe disease: Implications for motor unit dysfunction. Muscle Nerve 2015;51:877-83. [Crossref] [PubMed]
- Raben N, Nichols RC, Boerkoel C, et al. Genetic defects in patients with glycogenosis type II (acid maltase deficiency). Muscle Nerve Suppl 1995;3:S70-4. [Crossref] [PubMed]
- DeRuisseau LR, Fuller DD, Qiu K, et al. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci U S A 2009;106:9419-24. [Crossref] [PubMed]
- Byrne BJ, Falk DJ, Pacak CA, et al. Pompe disease gene therapy. Hum Mol Genet 2011;20:R61-8. [Crossref] [PubMed]
- Mah C, Cresawn KO, Fraites TJ Jr, et al. Sustained correction of glycogen storage disease type II using adeno-associated virus serotype 1 vectors. Gene Ther 2005;12:1405-9. [Crossref] [PubMed]
- Falk DJ, Soustek MS, Mah CS, et al. Systemic delivery of AAV2/9 vectors improve cardiac function in Pompe disease. American Society of Gene and Cell Therapy 13th Annual Meeting; Washington, DC; 2010.
- Sun B, Zhang H, Franco LM, et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther 2005;11:57-65. [Crossref] [PubMed]
- Raben N, Nagaraju K, Lee E, et al. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem 1998;273:19086-92. [Crossref] [PubMed]
- Pauly DF, Fraites TJ, Toma C, et al. Intercellular transfer of the virally derived precursor form of acid alpha-glucosidase corrects the enzyme deficiency in inherited cardioskeletal myopathy Pompe disease. Hum Gene Ther 2001;12:527-38. [Crossref] [PubMed]
- Amalfitano A, McVie-Wylie AJ, Hu H, et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc Natl Acad Sci U S A 1999;96:8861-6. [Crossref] [PubMed]
- Fraites TJ Jr, Schleissing MR, Shanely RA, et al. Correction of the enzymatic and functional deficits in a model of Pompe disease using adeno-associated virus vectors. Mol Ther 2002;5:571-8. [Crossref] [PubMed]
- Borger DK, McMahon B, Roshan Lal T, et al. Induced pluripotent stem cell models of lysosomal storage disorders. Dis Model Mech 2017;10:691-704. [Crossref] [PubMed]
- Chanana AM, Rhee JW, Wu JC. Human-induced pluripotent stem cell approaches to model inborn and acquired metabolic heart diseases. Curr Opin Cardiol 2016;31:266-74. [Crossref] [PubMed]
- Huang HP, Chen PH, Hwu WL, et al. Human Pompe disease-induced pluripotent stem cells for pathogenesis modeling, drug testing and disease marker identification. Hum Mol Genet 2011;20:4851-64. [Crossref] [PubMed]
- Kawagoe S, Higuchi T, Meng XL, et al. Generation of induced pluripotent stem (iPS) cells derived from a murine model of Pompe disease and differentiation of Pompe-iPS cells into skeletal muscle cells. Mol Genet Metab 2011;104:123-8. [Crossref] [PubMed]
- Nelson BC, Hashem SI, Adler ED. Human-Induced Pluripotent Stem Cell-Based Modeling of Cardiac Storage Disorders. Curr Cardiol Rep 2017;19:26. [Crossref] [PubMed]
- Bijvoet AG, van de Kamp EH, Kroos MA, et al. Generalized glycogen storage and cardiomegaly in a knockout mouse model of Pompe disease. Hum Mol Genet 1998;7:53-62. [Crossref] [PubMed]
- Seppälä EH, Reuser AJ, Lohi H. A nonsense mutation in the acid alpha-glucosidase gene causes Pompe disease in Finnish and Swedish Lapphunds. PLoS One 2013;8:e56825. [Crossref] [PubMed]
- Howell RR, Byrne B, Darras BT, et al. Diagnostic challenges for Pompe disease: an under-recognized cause of floppy baby syndrome. Genet Med 2006;8:289-96. [Crossref] [PubMed]
- Kishnani PS, Howell RR. Pompe disease in infants and children. J Pediatr 2004;144:S35-43. [Crossref] [PubMed]
- Hahn SH, Kronn D, Leslie ND, et al. Efficacy, safety profile, and immunogenicity of alglucosidase alfa produced at the 4,000-liter scale in US children and adolescents with Pompe disease: ADVANCE, a phase IV, open-label, prospective study. Genet Med 2018;20:1284-94. [Crossref] [PubMed]
- Burton BK, Kronn DF, Hwu WL, et al. The Initial Evaluation of Patients After Positive Newborn Screening: Recommended Algorithms Leading to a Confirmed Diagnosis of Pompe Disease. Pediatrics 2017;140:S14-23. [Crossref] [PubMed]
- Nicolino M, Byrne B, Wraith JE, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med 2009;11:210-9. [Crossref] [PubMed]
- Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 2007;68:99-109. [Crossref] [PubMed]
- van Capelle CI, Poelman E, Frohn-Mulder IM, et al. Cardiac outcome in classic infantile Pompe disease after 13years of treatment with recombinant human acid alpha-glucosidase. Int J Cardiol 2018;269:104-10. [Crossref] [PubMed]
- Mendelsohn NJ, Messinger YH, Rosenberg AS, et al. Elimination of antibodies to recombinant enzyme in Pompe's disease. N Engl J Med 2009;360:194-5. [Crossref] [PubMed]
- Mah C, Pacak CA, Cresawn KO, et al. Physiological correction of Pompe disease by systemic delivery of adeno-associated virus serotype 1 vectors. Mol Ther 2007;15:501-7. [Crossref] [PubMed]
- van Til NP, Stok M, Aerts Kaya FS, et al. Lentiviral gene therapy of murine hematopoietic stem cells ameliorates the Pompe disease phenotype. Blood 2010;115:5329-37. [Crossref] [PubMed]
- Raben N, Danon M, Gilbert AL, et al. Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab 2003;80:159-69. [Crossref] [PubMed]
- Pacak CA, Mah C, Cresawn KO, et al. rAAV2/9 Mediated Gene Delivery of Acid α-Glucosidase Corrects the Cardiac Phenotype in a Mouse Model of Pompe Disease. American Society of Gene Therapy 9th Annual Meeting; Baltimore, MD: Molecular Therapy; 2006.
- Pacak CA, Mah CS, Thattaliyath BD, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res 2006;99:e3-9. [Crossref] [PubMed]
- Cresawn KO, Fraites TJ, Wasserfall C, et al. Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum Gene Ther 2005;16:68-80. [Crossref] [PubMed]
- Rucker M, Fraites TJ Jr, Porvasnik SL, et al. Rescue of enzyme deficiency in embryonic diaphragm in a mouse model of metabolic myopathy: Pompe disease. Development 2004;131:3007-19. [Crossref] [PubMed]
- Sun B, Young SP, Li P, et al. Correction of multiple striated muscles in murine Pompe disease through adeno-associated virus-mediated gene therapy. Mol Ther 2008;16:1366-71. [Crossref] [PubMed]
- Sun B, Zhang H, Franco LM, et al. Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter. Mol Ther 2005;11:889-98. [Crossref] [PubMed]
- Ziegler RJ, Bercury SD, Fidler J, et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum Gene Ther 2008;19:609-21. [Crossref] [PubMed]
- Foley JW, Bercury SD, Finn P, et al. Evaluation of systemic follistatin as an adjuvant to stimulate muscle repair and improve motor function in Pompe mice. Mol Ther 2010;18:1584-91. [Crossref] [PubMed]
- Pacak CA, Sakai Y, Thattaliyath BD, et al. Tissue specific promoters improve specificity of AAV9 mediated transgene expression following intra-vascular gene delivery in neonatal mice. Genet Vaccines Ther 2008;6:13. [Crossref] [PubMed]
- Sun B, Li S, Bird A, et al. Antibody formation and mannose-6-phosphate receptor expression impact the efficacy of muscle-specific transgene expression in murine Pompe disease. J Gene Med 2010;12:881-91. [Crossref] [PubMed]
- Corti M, Cleaver B, Clement N, et al. Evaluation of Readministration of a Recombinant Adeno-Associated Virus Vector Expressing Acid Alpha-Glucosidase in Pompe Disease: Preclinical to Clinical Planning. Hum Gene Ther Clin Dev 2015;26:185-93. [Crossref] [PubMed]
- Doerfler PA, Nayak S, Corti M, et al. Targeted approaches to induce immune tolerance for Pompe disease therapy. Mol Ther Methods Clin Dev 2016;3:15053. [Crossref] [PubMed]
- Ahuja A, Shupe J, Dunn R, et al. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol 2007;179:3351-61. [Crossref] [PubMed]
- Doerfler PA, Nayak S, Herzog RW, et al. BAFF blockade prevents anti-drug antibody formation in a mouse model of Pompe disease. Clin Immunol 2015;158:140-7. [Crossref] [PubMed]
- Nayak S, Doerfler PA, Porvasnik SL, et al. Immune responses and hypercoagulation in ERT for Pompe disease are mutation and rhGAA dose dependent. PLoS One 2014;9:e98336. [Crossref] [PubMed]
- Corti M, Elder M, Falk D, et al. B-Cell Depletion is Protective Against Anti-AAV Capsid Immune Response: A Human Subject Case Study. Mol Ther Methods Clin Dev 2014.1. [PubMed]
- Raben N, Lu N, Nagaraju K, et al. Conditional tissue-specific expression of the acid alpha-glucosidase (GAA) gene in the GAA knockout mice: implications for therapy. Hum Mol Genet 2001;10:2039-47. [Crossref] [PubMed]
- Khanna R, Powe AC Jr, Lun Y, et al. The pharmacological chaperone AT2220 increases the specific activity and lysosomal delivery of mutant acid alpha-glucosidase, and promotes glycogen reduction in a transgenic mouse model of Pompe disease. PLoS One 2014;9:e102092. [Crossref] [PubMed]
- Mah CS, Falk DJ, Germain SA, et al. Gel-mediated delivery of AAV1 vectors corrects ventilatory function in Pompe mice with established disease. Mol Ther 2010;18:502-10. [Crossref] [PubMed]
- Puzzo F, Colella P, Biferi MG, et al. Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid alpha-glucosidase. Sci Transl Med 2017;9. [Crossref] [PubMed]
- Colella P, Sellier P, Costa Verdera H, et al. AAV Gene Transfer with Tandem Promoter Design Prevents Anti-transgene Immunity and Provides Persistent Efficacy in Neonate Pompe Mice. Mol Ther Methods Clin Dev 2018;12:85-101. [Crossref] [PubMed]
- Mah C, Fraites TJ Jr, Cresawn KO, et al. A new method for recombinant adeno-associated virus vector delivery to murine diaphragm. Mol Ther 2004;9:458-63. [Crossref] [PubMed]
- Turner SM, Hoyt AK, ElMallah MK, et al. Neuropathology in respiratory-related motoneurons in young Pompe (Gaa(-/-)) mice. Respir Physiol Neurobiol 2016;227:48-55. [Crossref] [PubMed]
- Turner SMF, Falk DJ, Byrne BJ, et al. Transcriptome assessment of the Pompe (Gaa-/-) mouse spinal cord indicates widespread neuropathology. Physiol Genomics 2016;48:785-94. [Crossref] [PubMed]
- Lee NC, Hwu WL, Muramatsu SI, et al. A Neuron-Specific Gene Therapy Relieves Motor Deficits in Pompe Disease Mice. Mol Neurobiol 2018;55:5299-309. [Crossref] [PubMed]
- Raben N, Plotz P, Byrne BJ. Acid alpha-glucosidase deficiency (glycogenosis type II, Pompe disease). Curr Mol Med 2002;2:145-66. [Crossref] [PubMed]
- Gambetti P, DiMauro S, Baker L. Nervous system in Pompe's disease. Ultrastructure and biochemistry. J Neuropathol Exp Neurol 1971;30:412-30. [Crossref] [PubMed]
- Mancall EL, Aponte GE, Berry RG. Pompe's Disease (Diffuse Glycogenosis) with Neuronal Storage. J Neuropathol Exp Neurol 1965;24:85-96. [Crossref] [PubMed]
- Martin JJ, de Barsy T, van Hoof F, et al. Pompe's disease: an inborn lysosomal disorder with storage of glycogen. A study of brain and striated muscle. Acta Neuropathol 1973;23:229-44. [Crossref] [PubMed]
- Martini C, Ciana G, Benettoni A, et al. Intractable fever and cortical neuronal glycogen storage in glycogenosis type 2. Neurology 2001;57:906-8. [Crossref] [PubMed]
- Teng YT, Su WJ, Hou JW, et al. Infantile-onset glycogen storage disease type II (Pompe disease): report of a case with genetic diagnosis and pathological findings. Chang Gung Med J 2004;27:379-84. [PubMed]
- Matsui T, Kuroda S, Mizutani M, et al. Generalized glycogen storage disease in Japanese quail (Coturnix coturnix japonica). Vet Pathol 1983;20:312-21. [Crossref] [PubMed]
- Sidman RL, Taksir T, Fidler J, et al. Temporal neuropathologic and behavioral phenotype of 6neo/6neo Pompe disease mice. J Neuropathol Exp Neurol 2008;67:803-18. [Crossref] [PubMed]
- Clement DH, Godman GC. Glycogen disease resembling mongolism, cretinism, and amytonia congenita; case report and review of literature. J Pediatr 1950;36:11-30, illust. [Crossref] [PubMed]
- Hogan GR, Gutmann L, Schmidt R, et al. Pompe's disease. Neurology 1969;19:894-900. [Crossref] [PubMed]
- Willemsen MA, Jira PE, Gabreëls FJ, et al. Three hypotonic neonates with hypertrophic cardiomyopathy: Pompe's disease. Ned Tijdschr Geneeskd 1998;142:1388-92. [PubMed]
- Zellweger H, Dark A, Abu-Haidar GA. Glycogen disease of skeletal muscle; report of two cases and review of literature. Pediatrics 1955;15:715-32. [PubMed]
- Rohrbach M, Klein A, Kohli-Wiesner A, et al. CRIM-negative infantile Pompe disease: 42-month treatment outcome. J Inherit Metab Dis 2010;33:751-7. [Crossref] [PubMed]
- ElMallah MK, Falk DJ, Lane MA, et al. Retrograde gene delivery to hypoglossal motoneurons using adeno-associated virus serotype 9. Hum Gene Ther Methods 2012;23:148-56. [Crossref] [PubMed]
- Elmallah MK, Falk DJ, Nayak S, et al. Sustained correction of motoneuron histopathology following intramuscular delivery of AAV in pompe mice. Mol Ther 2014;22:702-12. [Crossref] [PubMed]
- Falk DJ, Mah CS, Soustek MS, et al. Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease. Mol Ther 2013;21:1661-7. [Crossref] [PubMed]
- Falk DJ, Todd AG, Lee S, et al. Peripheral nerve and neuromuscular junction pathology in Pompe disease. Hum Mol Genet 2015;24:625-36. [Crossref] [PubMed]
- Lee KZ, Qiu K, Sandhu MS, et al. Hypoglossal neuropathology and respiratory activity in pompe mice. Front Physiol 2011;2:31. [Crossref] [PubMed]
- Todd AG, McElroy JA, Grange RW, et al. Correcting Neuromuscular Deficits With Gene Therapy in Pompe Disease. Ann Neurol 2015;78:222-34. [Crossref] [PubMed]
- Kikuchi T, Yang HW, Pennybacker M, et al. Clinical and metabolic correction of pompe disease by enzyme therapy in acid maltase-deficient quail. J Clin Invest 1998;101:827-33. [Crossref] [PubMed]
- Van den Hout JM, Kamphoven JH, Winkel LP, et al. Long-term intravenous treatment of Pompe disease with recombinant human alpha-glucosidase from milk. Pediatrics 2004;113:e448-57. [Crossref] [PubMed]
- Muller CW, Jones HN, O'Grady G, et al. Language and speech function in children with infantile Pompe disease. J Pediatr Neurol 2009;7:147-56.
- McIntosh PT, Hobson-Webb LD, Kazi ZB, et al. Neuroimaging findings in infantile Pompe patients treated with enzyme replacement therapy. Mol Genet Metab 2018;123:85-91. [Crossref] [PubMed]
- Doerfler PA, Todd AG, Clement N, et al. Copackaged AAV9 Vectors Promote Simultaneous Immune Tolerance and Phenotypic Correction of Pompe Disease. Hum Gene Ther 2016;27:43-59. [Crossref] [PubMed]
- ElMallah MK, Pagliardini S, Turner SM, et al. Stimulation of Respiratory Motor Output and Ventilation in a Murine Model of Pompe Disease by Ampakines. Am J Respir Cell Mol Biol 2015;53:326-35. [Crossref] [PubMed]
- Falk DJ, Soustek MS, Todd AG, et al. Comparative impact of AAV and enzyme replacement therapy on respiratory and cardiac function in adult Pompe mice. Mol Ther Methods Clin Dev 2015;2:15007. [Crossref] [PubMed]
- Lowenstein PR, Mandel RJ, Xiong WD, et al. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther 2007;7:347-60. [Crossref] [PubMed]
- Hester ME, Foust KD, Kaspar RW, et al. AAV as a gene transfer vector for the treatment of neurological disorders: novel treatment thoughts for ALS. Curr Gene Ther 2009;9:428-33. [Crossref] [PubMed]
- Qiu K, Falk DJ, Reier PJ, et al. Spinal delivery of AAV vector restores enzyme activity and increases ventilation in Pompe mice. Mol Ther 2012;20:21-7. [Crossref] [PubMed]
- Herbert M, Case LE, Rairikar M, et al. Early-onset of symptoms and clinical course of Pompe disease associated with the c.-32-13T>G variant. Mol Genet Metab 2019;126:106-16. [Crossref] [PubMed]
- Musumeci O, Marino S, Granata F, et al. Central nervous system involvement in late-onset Pompe disease: clues from neuroimaging and neuropsychological analysis. Eur J Neurol 2019;26:442-e35. [Crossref] [PubMed]
- Figueroa-Bonaparte S, Llauger J, Segovia S, et al. Quantitative muscle MRI to follow up late onset Pompe patients: a prospective study. Sci Rep 2018;8:10898. [Crossref] [PubMed]
- Ebbink BJ, Poelman E, Aarsen FK, et al. Classic infantile Pompe patients approaching adulthood: a cohort study on consequences for the brain. Dev Med Child Neurol 2018;60:579-86. [Crossref] [PubMed]
- Tarallo A, Carissimo A, Gatto F, et al. microRNAs as biomarkers in Pompe disease. Genet Med 2019;21:591-600. [Crossref] [PubMed]
- Kishnani PS, Goldenberg PC, DeArmey SL, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 2010;99:26-33. [Crossref] [PubMed]
- Sun B, Kulis MD, Young SP, et al. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine pompe disease. Mol Ther 2010;18:353-60. [Crossref] [PubMed]
- Nayak S, Cao O, Hoffman BE, et al. Prophylactic immune tolerance induced by changing the ratio of antigen-specific effector to regulatory T cells. J Thromb Haemost 2009;7:1523-32. [Crossref] [PubMed]
- LoDuca PA, Hoffman BE, Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther 2009;9:104-14. [Crossref] [PubMed]
- Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther 2010;17:295-304. [Crossref] [PubMed]
- Martino AT, Nayak S, Hoffman BE, et al. Tolerance induction to cytoplasmic beta-galactosidase by hepatic AAV gene transfer: implications for antigen presentation and immunotoxicity. PLoS One 2009;4:e6376. [Crossref] [PubMed]
- Hoffman BE, Martino AT, Sack BK, et al. Nonredundant roles of IL-10 and TGF-β in suppression of immune responses to hepatic AAV-factor IX gene transfer. Mol Ther 2011;19:1263-72. [Crossref] [PubMed]
- Herzog RW, Cooper M, Perrin GQ, et al. Regulatory T cells and TLR9 activation shape antibody formation to a secreted transgene product in AAV muscle gene transfer. Cell Immunol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Calcedo R, Vandenberghe LH, Gao G, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199:381-90. [Crossref] [PubMed]
- Cao O, Hoffman BE, Moghimi B, et al. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther 2009;17:1733-42. [Crossref] [PubMed]
- Cooper M, Nayak S, Hoffman BE, et al. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum Gene Ther 2009;20:767-76. [Crossref] [PubMed]
- Joseph A, Munroe K, Housman M, et al. Immune tolerance induction to enzyme-replacement therapy by co-administration of short-term, low-dose methotrexate in a murine Pompe disease model. Clin Exp Immunol 2008;152:138-46. [Crossref] [PubMed]
- Smith BK, Collins SW, Conlon TJ, et al. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gene Ther 2013;24:630-40. [Crossref] [PubMed]
- Byrne PI, Collins S, Mah CC, et al. Phase I/II trial of diaphragm delivery of recombinant adeno-associated virus acid alpha-glucosidase (rAAaV1-CMV-GAA) gene vector in patients with Pompe disease. Hum Gene Ther Clin Dev 2014;25:134-63. [Crossref] [PubMed]
- Byrne BJ, Kishnani PS, Case LE, et al. Pompe Disease: Design, methodology, and early findings from the Pompe Registry. Mol Genet Metab 2011;103:1-11. [Crossref] [PubMed]


