
Smoking favours hepatocellular carcinoma
Smoking and liver cancer
Chronic hepatitis B or C virus (HBV or HCV) remained the strongest risk factor for hepatocellular carcinoma (HCC), but results of a nested case-control study in Europe showed that 47.6% of HCC was associated with smoking (1). The International Agency for Research on Cancer (IARC) showed enough evidence to classify liver cancer as a tobacco related malignancy in 2004 (2). The available knowledge on the relationship between tobacco smoking and a variety of cancers is based primarily on epidemiological evidences. Results from a meta-analysis of 81 epidemiological studies supported this association, with significant pooled ORs for HCC development of 1.55 (95% CI, 1.46–1.65) and 1.39 (95% CI, 1.26–1.52) for current and former smokers, respectively, compared to non-smokers. They identified also a dose-response effect with a significant increase in liver cancer risk for heavy smokers (OR 1.9; 95% CI, 1.68–2.14) (3). Confirming these data, Schulze et al. identified 8 HCC-risk-factor-specific mutational signatures, and two of them (MSig1 and MSig3) associated with tobacco exposure, suggesting a genotoxic effect of smoking. More interesting they found also a hypermutated tumor (classified alone, outside the previous signatures) characterized by a non-fibrotic liver with black anthracotic pigment deposition and by a predominance of C>T mutations resulting from the interplay between an unknown mutagenic process influencing particularly guanine bases and transcription coupled nucleotide excision repair (4). An impact of smoking on HCC development is possible and have biological substrate, according to the carcinogenic potential of various molecules with oncogenic potential such as 4-aminobiphenyl, a liver carcinogen which has been identified as a causal risk factor for liver cancer. These oncogenic compounds covalently bind to DNA creating DNA adducts, which have a central role in the carcinogenesis by causing miscoding events in critical genes (5). Smoking increases oxidative stress and release of pro-inflammatory cytokines causing inflammation (6).
Synergic effect of smoking and HBV infection
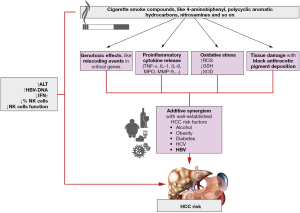
It has been also highlighted that smoking may be associated with increased HCC risk in association with other established HCC risk factors, like alcohol, obesity, diabetes and other metabolic risk factors (7). Additionally, additive synergism between smoking and HBV/HCV infection on HCC risk was showed by a recent meta-analysis of prospective cohort studies (8) (Figure 1). Moreover, Kolly et al. showed that smokers have a significant worse overall survival than non-smokers (HR 1.77, 95% CI, 1.22–2.58), particularly in the setting of viral hepatitis in which smoking is an independent predictor for survival (HR 2.99; 95% CI, 1.75–5.23) (9). Recently Wang et al. revealed the relationship between smoking and the development of HBV-related HCC, highlighting a role of smoking on HBV viral load and liver inflammation (10). They used two approaches: a nested case-control study assessing the mediation effect of alanine aminotransferase (ALT) levels and viral load on the association between smoking and HCC risk, and a longitudinal analysis assessing the effects of smoking on ALT and viral load, evaluating if this association was in accordance with long-lasting immunity to infection. They noticed a dose-related effect of cumulative smoking on ALT and viral load. An association between smoking and ALT levels has been already described among HCV-infected individuals (11). They showed that smoking is associated with an adjusted OR of 1.68 for de novo HCC events, and the OR falls to 1.37 after adjusting for viral load. A mediation analyses highlighted that viral load mediates 31.7% of the effects of smoking on HCC risk while ALT alone is not a significant mediator.
Smoking and immune response
What is innovative in this paper is the attempt to achieve a better understanding of the effects of smoking on HBV viral load and ALT through the assessment of plasma levels of interferon (IFN)γ and of cellular immune and transcriptional profiles. They observed a dose-dependent effect of cumulative smoking exposure on IFNγ levels and on reduced percentage and function of natural killer (NK) cells in peripheral blood (Figure 1). Finally, they found that the combination of smoking and the decrease of NK cell fraction below 22% is associated with increased likelihood of occurring ALT ≥80 U/L and higher viral load. It was demonstrated that increased viral load mediated by smoking would induce hepatic inflammation and abnormal immunity, which has close correlation with the development of HBV-related HCC.
Future investigations
The role of elevated ALT and high viral load in HBV-related HCC is well known, and what is missing in this analysis is the effect of antiviral therapy (nucleotide/nucleoside analogues) on smoking consequences, because antiviral drugs have long-term beneficial impact on natural course of HBV infection in terms of liver decompensation, HCC occurrence and overall survival (12). Future investigations should take into account the impact of antiviral therapy on liver disease progression, and probably a study cohort comparing subgroups of patients according to smoking/not smoking/smoking cessation ± nucleotide/nucleoside analogues therapy is mandatory to investigate if smoking cessation or not smoking at all can have a synergic effect with antiviral therapy in terms of disease progression, HCC development and mortality. Additionally, analysis of NK cells, at key time-points of the natural history of the liver disease and according to smoking habits, could be helpful to better understanding the immunological mechanisms involved on the development of HBV-related HCC in smokers vs. not smokers. These findings underline the importance of smoking cessation and prevention in hepatitis B management, since the increased risk of HCC in smokers is mediated by carcinogens-induced genetic changes and immunological effects on persistent viral infection and hepatocellular damage.
Conclusions
In conclusion, it is important to counsel and encourage all patients who have risk factors for HCC or patients already diagnosed with HCC to stop smoking, explaining them the potential deleterious effects on chronic liver disease and survival if they continue smoking. More efforts are required to promote smoking cessation in the overall population and specially in patients with chronic liver diseases.
Acknowledgments
None.
Footnote
Conflicts of Interest: JF Dufour: Advisory committee for Abbvie, Bayer, BMS, Fallk, Genfit, Genkyotex, Gilead, Heparegenerix, Intercept, Lilly, Merck, Novartis. M Guarino has no conflicts of interest to declare.
References
- Trichopoulos D, Bamia C, Lagiou P, et al. Hepatocellular carcinoma risk factors and disease burden in a European cohort: a nested case-control study. J Natl Cancer Inst 2011;103:1686-95. [Crossref] [PubMed]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 83. Lyon, France: IARC, 2004.
- Abdel-Rahman O, Helbling D, Schöb O, et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: An updated systematic review of 81 epidemiological studies. J Evid Based Med 2017;10:245-54. [Crossref] [PubMed]
- Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet 2015;47:505-11. [Crossref] [PubMed]
- Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer 2012;131:2724-32. [Crossref] [PubMed]
- Altamirano J, Bataller R. Cigarette smoking and chronic liver diseases. Gut 2010;59:1159-62. [Crossref] [PubMed]
- Yu MW, Lin CL, Liu CJ, et al. Influence of Metabolic Risk Factors on Risk of Hepatocellular Carcinoma and Liver-Related Death in Men With Chronic Hepatitis B: A Large Cohort Study. Gastroenterology 2017;153:1006-17.e5. [Crossref] [PubMed]
- Chuang SC, Lee YC, Hashibe M, et al. Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2010;19:1261-8. [Crossref] [PubMed]
- Kolly P, Knöpfli M, Dufour JF. Effect of smoking on survival of patients with hepatocellular carcinoma. Liver Int 2017;37:1682-7. [Crossref] [PubMed]
- Wang YH, Chuang YH, Wu CF, et al. Smoking and Hepatitis B Virus-Related Hepatocellular Carcinoma Risk: The Mediating Roles of Viral Load and Alanine Aminotransferase. Hepatology 2019;69:1412-25. [Crossref] [PubMed]
- Wang CS, Wang ST, Chang TT, et al. Smoking and alanine aminotransferase levels in hepatitis C virus infection: implications for prevention of hepatitis C virus progression. Arch Intern Med 2002;162:811-5. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-98. [Crossref] [PubMed]


