
Perineural invasion in cervical cancer: pay attention to the indications of nerve-sparing radical hysterectomy
Introduction
In 1898, Ernst Wertheim first introduced the radical abdominal hysterectomy for women with cervical cancer and over time, the procedure was named after him (1). Subsequently, radical hysterectomy and pelvic lymphadenectomy with or without paraaortic nodal sampling is widely used for the treatment of early-stage cervical cancer. However, a conventional radical hysterectomy with extensive parametrial resection can be performed if adequate tumor-free margins are available. Moreover, the procedure was shown to cause damage to the pelvic autonomic nerves leading to difficulties in storage and voiding urine, colorectal disorders, and sexual dysfunction. Additionally, the physical and mental stress was found to impair the quality of life of the patients (2).
In order to avoid long-term adverse effects, nerve-sparing radical hysterectomy (NSRH) was introduced by Japanese surgeons to preserve the pelvic autonomic nerves. A recent meta-analysis demonstrated that NSRH linked to reduced bladder dysfunctions and fewer postoperative complications (3). NSRH is not only a feasible and well-tolerated procedure, but might also improve the quality of life (4). However, there is no standardized technique for NSRH. Recently, it has been found that cervical cancer exhibited a tendency towards neural invasion (5). This neoplastic invasion of nerves is called perineural invasion (PNI). As PNI is correlated with poor prognosis in cervical cancer, we need to pay attention to strictly control the NSRH indications. It is important to balance the treatment and quality of life of patients, an essential aspect for future developments in surgical techniques for cervical cancer. Further understanding of PNI may open doors to formulate NSRH indication and standardize surgical procedures.
Anatomical bases and definition of PNI
PNI is also called neurotropic carcinomatous spread or perineural spread (6). As the fifth route of cancer spread, which is different from transcoelomic, lymphatic spread, hematogenous spread, and canalicular spread. Nevertheless, PNI is frequently disregarded and is not well understood.
There are three layers of connective tissue covering each nerve, namely the outermost epineurium, the middle perineurium, and the innermost endoneurium (7). The inside part of the nerve is separated from the surrounding tumor by multiple layers of collagen and basement membrane. This anatomical structure serves as a low-resistance plane that provides channels to the tumor cells for spreading along the neural sheaths. Once the tumor cells have invaded the nerve sheath, it may access the growth environment that is beneficial for metastasis. This was the predominant theory for the last 40 years. Through continuous pathological section analysis, it was found that the transfer mode of PNI was continuous, non-jumping, and direct spreading. Thus, tumor cells migrate through a “neural road”.
The patterns of invasion of neuron widely varies, including complete and incomplete encirclement, concentric lamination, and tangential contact (8). A more common exist in PNI is tumor cells free inside of the nerve sheath but tumor-nerve contact within the perineurium (9). The current pathological diagnostic criteria of PNI is “tumor in close proximity to nerve and involving at least 33% of its circumference or tumor cells within any of the 3 layers of the nerve sheath” (9). In fact, there is no agreement regarding the interpretation of PNI among pathologists who conduct microscopic examination of tissue specimens (10). Thus, the conventional H & E stained section and immunohistochemical examination of biomarkers of autonomic nerves and cancer cells will be necessary. Furthermore, it is necessary to establish a risk model exploring the relationship between nerve-tumor distance and nerve diameter with clinical outcomes (11). These examinations will definitely form the basis of radical hysterectomy in the era of precision surgery.
Mechanisms of PNI
In the mid-1800s, researches in the field of head and neck cancers first reported PNI. They showed that these tumors exhibited a predilection for growth along nerves leading their way to the intracranial fossa. However, at the time, the molecular mechanism of certain carcinomas predilection for PNI was largely unknown. Traditionally, tumor propagation occurs along the perineural space, which has a low resistance depending on the connective tissue covering the peripheral nerve (12). Nevertheless, recent studies including animal models and human tissues have demonstrated that cancer cells have an innate nature to migrate along axons in a mechanism named neural tracking, thereby challenging the conventional knowledge. To migrate along the nerves, the cancer cells need the support of multiple growth factors and chemokines secreted by various types of cell in the perineural niche (13). New evidences suggest a complex interaction between nerves and tumor cells invading the nerves; signal transduction through neurotrophic growth factors such as neurotropin, granulocyte colony-stimulating factor (G-CSF), and cytokines has been observed (14-16). Neurotrophins and their receptors are being investigated as viable therapeutic targets (17-20). Moreover, researchers have focused on the genetic mechanism of PNI including gene defect and the role of tumor suppressor gene (p73) (21).
Sympathetic nervous system regulates the tumor microenvironment. The cervix and uterus are innervated by the autonomic nervous system (ANS) (22). Activation of the sympathetic division of the ANS in particular modulates gene expression that promotes metastasis of solid tumors (23). A tumor is able to induce neoneurogenesis; high levels of nerve growth factor and axon guidance molecules have been observed in the presence of a tumor (24). High levels of norepinephrine released by the sympathetic nerves contribute to tumor development and metastasis, indicating a mutually beneficial relationship between tumor cells and neurons (25). Recent research demonstrates that Schwann cells have a unique and specific affinity for tumor, and aid in tumor dissociation, migration, and invasion (26-28). Neural cell adhesion molecule 1 (NCAM1) is an important molecular mediator of Schwann cell directed PNI (25,29). During PNI, there is communication between nerves and cancer cells. The new findings have demonstrated that cancer invasion is promoted by prostaglandin E2. Following the feedback mechanism, which Galanin (GAL) released by cancer cells induces neuritogenesis, facilitating PNI (30). Nerves and cancer cells are like two “waltz” dancers going to each other, and eventually lead to tumor cells invading and spreading. The tumor cells can use splanchnic nerves as conduits and spread from the end organ to the lumbosacral plexus (31). Epidemiological studies and preclinical trials suggested that the nervous system plays an important role in tumorigenesis, and that denervation might reduce or slow down tumor progression and PNI (32-34).
PNI in cervical cancer and clinical significance
PNI has been shown to be an important pathological feature of cervical cancer and along with cancer of other organs, including head and neck, pancreas, colon, rectum, prostate, biliary tract, and stomach (35-39). PNI is related to morbidity and play a key role in the poor outcome and overall survival of the patients.
The treatment of early-stage cervical cancer includes radical hysterectomy and pelvic lymph node dissection, followed by neoadjuvant chemotherapy (NACT) if necessary. This can achieve 5-year survival rates of approximately 85% (40-42). Additional adjuvant treatment is considered based on the risk factors of recurrence. Well-known high-risk factors such as lymph node metastasis, parametrial invasion and resection margin involvement could increase the recurrence rate ≤40% in postoperative cervical cancer (43,44). Intermediate-risk factors include tumor size, depth of stromal invasion, and lymphovascular space invasion (LVSI) (45,46). However, so far, numerous large-scale studies in terms of morphologic parameters do not recognize PNI (47-52), and the histopathologic description of parametria has usually ignored the existence of PNI.
Table 1 summarizes the studies of PNI in early-stage cervical cancer, including its incidence, coexistence with other pathological and clinical features, as well as its prognostic value. In previous studies, PNI was observed in 7.0–35.1% patients with early-stage cervical cancer (53-60). Unfortunately, some patients in these studies received NACT before the operations, and the rate of PNI in operated samples was thought to significantly decrease. Future research should focus on standardizing the definition, reporting methods as well as histopathology of relevant nerve-specific antigens.
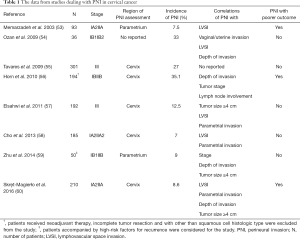
Full table
PNI was significantly associated with multiple high risk and intermediate-risk factors for recurrence (Table 1), especially, larger tumors (≥4 cm), depths of invasion (≥15 mm), and LVSI, which play a crucial part in deciding the adjuvant therapy. A previous study has shown that more than half of the patients with PNI (75.0–92.3%) also had evidence of LVSI, compared with 7.7–39.9% of those who had no PNI. Memarzadeh et al. (53) suggested the spread of malignancy from primary cervical tumor to parametrium in the form of LVSI or parametrial PNI with subsequent involvement of the lymphatic channels with tumor cells. Larger tumor (≥4 cm) as well as deeper invasion (≥15 mm) were more common in PNI-positive than in PNI-negative tumors. Moreover, some studies reported that PNI was associated with other risk factors as well (Table 1). It seemed to imply that PNI was associated with more aggressive diseases. In previous studies conducted by Elsahwi et al. (57) and Cho et al. (58), 70–90% cervical cancer patients with PNI were recommended for adjuvant radiotherapy, which is significantly higher than that for patients without PNI. In cervical cancer, PNI was not an isolated event. Particularly, PNI was significantly associated with LVSI and larger tumors (≥4 cm). From the existing literature reports, there must be some high-risk factors for recurrence when PNI presents in cervical cancer. But, not vice versa, the existence of high-risk factors are not equal to PNI. There is no relevant report on the rate of occurrence PNI present independent of the presence of cancer. Multicenter, randomized, prospective trials are needed to further confirm these observations.
The clinical significance of PNI as a potential prognostic factor for cervical cancer has not been well studied. Memarzadeh et al. (53) and Horn et al. (56) demonstrated that the presence of PNI proved to be an independent predicting factor of poor outcome in patients with cervical cancer. Skręt-Magierło et al. (60) found that patients with PNI had shorter disease-free and overall survival; however, PNI was not identified as an independent risk factor. Contrary to these studies, others demonstrated that PNI did not increase the risk for recurrence or death. Five of eight studies shown in Table 1 did not consider PNI as a risk factor. However, these differences were not surprising because the subjects and surgical procedures widely varied in these studies. Recently, a meta-analysis, including three studies and 571 patients, demonstrated that PNI is closely related to the risk factors for recurrence and is an ominous morphologic prognostic factor in cervical cancer (61). Unfortunately, the sample number was too small and two of these three studies did not provide a direct hazard ratio.
PNI may be a new intermediate-risk factor for patients with cervical cancer, which is helpful to determine the strategy for providing adjuvant treatment to the patient. Adjuvant therapy would improve overall survival and disease-free survival. At this point, the use of PNI as an independent predictor for prognosis is limited. Further studies are required for estimating the prognostic value of PNI in cervical cancer.
Indication of NSRH
Currently, NSRH is an important topic in clinical research. Studies demonstrate the cure rates for cervical cancer are equivalent to those of the conventional technique, which can also decrease vesical, rectal, and to a lesser degree, sexual dysfunction. However, for this surgical procedure, the postoperative recurrence rate is still unclear, and it lacks a standardized research methodology. Recently, three meta-analyses on NSRH criticized that there was no standardized technique for NSRH and controversies still exist about its safety in patients with cancer (3,62,63). In the presence of PNI, NSRH may preserve not only the nerve but also the cancer cells invading the nerves. Therefore, it must be very strict to NSRH indications and pay attention to PNI, which is particularly important. Perineural spread increases the difficulties of completely removing the tumor with safe margins at the time of diagnosis. Therefore, optimal resection in such cases is rare (64,65).
PNI was correlated with the well-known risk factors. Furthermore, PNI may be present in the parametrium in early-stage cervical cancer, so NSRH may retain the risk of recurrence (or residual tumor). High-risk factors including PNI should be excluded by preoperative examinations to ascertain the eligibility criteria for performing of NSRH. At present, PNI is diagnosed by pain symptoms, magnetic resonance imaging (MRI), and examination of intraoperative frozen sections. In 60% of the patients with malignant tumor of the pelvis have been shown to experience a neuropathic pain. The infiltration of the perineal nerves results in lumbosacral plexopathies and complete destruction of the nerve, which may be a reason for the pelvic pain (66). Because the early symptoms of cervical cancer are mild, it is easy to misdiagnose and often ignore the underlying PNI.
It has been reported that PNI may be detected by preoperative imaging studies using computed tomography (CT) or MRI and histologic evaluation (67,68). PNI evaluation by 3.0 high-field MRI may map the exact location of the tumor and improve critical surgical and treatment planning (69). Gil et al. (70) reported that PNI of the tumor cells transfected with NV1066, could be detected using stereo microscope or positron-emission tomography (PET) imaging. However, these findings were observed in oral squamous cell carcinoma, cutaneous squamous cell carcinoma, pancreatic cancer, prostate carcinoma, and adenoid cystic carcinoma, but data on cervical cancer are scarce or even nonexistent. Although still controversial, the information in literature regarding other types of cancer should be used as a reference for preoperative diagnosis of PNI in cervical cancer. Recently, Howe et al. reported that perineural spread in a case of cervical cancer to the sciatic nerve or a place 15 cm away could be detected by MRI (5). Thus, preoperative imaging is valuable for the detection of PNI, and could be used as a diagnostic method of PNI.
Previous studies have shown that when patients had a tumor sized <2 cm, there was no lymphovascular invasion, and the depth of invasion was limited. The risk of parametrial involvement is nearly 1% (62,71). We can use these favorable preoperative features to select patients who would receive maximum benefit from NSRH; subsequent postoperative radiation can also be avoided. During NSRH, the lower hypogastric plexus, vesical plexus, and rectal branches are saved, but the uterine branches are dissected. Skręt-Magierło et al. demonstrated that examination of an intraoperative frozen section of small slices of the uterine branches may be performed in the presence of high-risk factors including tumor size of ≥40 mm, depth of invasion of ≥15 mm, and/or pelvic or paraaortic lymph nodes identified using CT scans (60). Based on intraoperative frozen section examination, the procedure of nerve sparing can continue if the sample is free from invasion. This approach is difficult to clear the status of nerve trunk, so it seems to be great risk. There are still some issues that need to be addressed in the intraoperative frozen section examination, for example: where should be examined? How many sites should be examined? How can the diagnostic accuracy be guaranteed in case of such intraoperative exam with frozen samples? Simultaneous identification of HPV 16 E6 and S100 by double immunofluorescence may be helpful to detect PNI in cervical cancer (72,73).
NSRH can be subdivided into unilateral nerve-sparing radical hysterectomies (UNSRH) and bilateral nerve-sparing radical hysterectomies (BNSRH) (74). The therapeutic effect of BNSRH is better than UNSRH. Therefore, patients with International Federation of Gynecology and Obstetrics (FIGO) stage IA2 and IB1 (<2 cm) cervical cancer could undergo BNSRH; however, to ensure the efficacy, UNSRH is needed for FIGO stage IIA1 (<2 cm and small lesion in the vagina vault) patients. The patients with FIGO stage IB2 and IIA2 who underwent NACT are not suitable for performing NSRH, since NACT may mask the occurrence of PNI. In addition, patients with the lowest possibility of postoperative radiotherapy may gain maximum benefit from NSRH in terms of quality of life.
Conclusions
There is no doubt that PNI should be regarded as one of the main factors affecting NSRH indications for cervical cancer. The aim to study PNI in cervical cancer is to develop clear indications of NSRH, and not to deny this surgical technique. The advantages of NSRH can be reflected more perfectly. It is important to ensure the survival of patients as well as, to improve the quality of life. Further multi-center and large prospective trials are needed to define the recommendations and guidelines for conducting NSRH.
Acknowledgements
Funding: This project was supported by the Grant-in-Aid for Scientific Research from Sichuan Government and Sichuan Applied Basic Research Project from Sichuan Provincial Science and Technology Department (2017JY0300).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Roh JW, Lee DO, Suh DH, et al. Efficacy and oncologic safety of nerve-sparing radical hysterectomy for cervical cancer: a randomized controlled trial. J Gynecol Oncol 2015;26:90-9. [Crossref] [PubMed]
- de Kroon CD, Gaarenstroom KN, van Poelgeest MI, et al. Nerve sparing in radical surgery for early-stage cervical cancer: yes we should! Int J Gynecol cancer 2010;20:S39-41. [Crossref] [PubMed]
- Long Y, Yao DS, Pan XW, et al. Clinical efficacy and safety of nerve-sparing radical hysterectomy for cervical cancer: a systematic review and meta-analysis. PloS one 2014;9:e94116. [Crossref] [PubMed]
- Sakuragi N. Nerve-sparing radical hysterectomy: time for a new standard of care for cervical cancer? J Gynecol Oncol 2015;26:81-2. [Crossref] [PubMed]
- Howe BM, Amrami KK, Nathan MA, et al. Perineural spread of cervical cancer to the sciatic nerve. Skeletal Radiol 2013;42:1627-31. [Crossref] [PubMed]
- Bilici A, Seker M, Ustaalioglu BB, et al. Prognostic significance of perineural invasion in patients with gastric cancer who underwent curative resection. Ann Surg Oncol 2010;17:2037-44. [Crossref] [PubMed]
- Scheuermann DW, Krammer HJ, Timmermans JP, et al. Fine structure of morphologically well-defined type II neurons in the enteric nervous system of the porcine small intestine revealed by immunoreactivity for calcitonin gene-related peptide. Acta Anat (Basel) 1991;142:236-41. [Crossref] [PubMed]
- Fagan JJ, Collins B, Barnes L, et al. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 1998;124:637-40. [Crossref] [PubMed]
- Liebig C, Ayala G, Wilks JA, et al. Perineural invasion in cancer: a review of the literature. Cancer 2009;115:3379-91. [Crossref] [PubMed]
- Chi AC, Katabi N, Chen HS, et al. Interobserver variation among pathologists in evaluating perineural invasion for oral squamous cell carcinoma. Head Neck Pathol 2016;10:451-64. [Crossref] [PubMed]
- Schmitd LB, Beesley LJ, Nickole R, et al. Redefining perineural invasion: integration of biology with clinical outcome. Neoplasia 2018;20:657-67. [Crossref] [PubMed]
- Panizza B, Warren TA, Solares CA, et al. Histopathological features of clinical perineural invasion of cutaneous squamous cell carcinoma of the head and neck and the potential implications for treatment. Head Neck 2014;36:1611-8. [Crossref] [PubMed]
- Amit M, Na'ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nat Rev Cancer 2016;16:399-408. [Crossref] [PubMed]
- Ketterer K, Rao S, Friess H, et al. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin Cancer Res 2003;9:5127-36. [PubMed]
- Marchesi F, Piemonti L, Fedele G, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res 2008;68:9060-9. [Crossref] [PubMed]
- Schweizerhof M, Stosser S, Kurejova M, et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nature Med 2009;15:802-7. [Crossref] [PubMed]
- Miknyoczki SJ, Lang D, Huang L, et al. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J cancer 1999;81:417-27. [Crossref] [PubMed]
- Papatsoris AG, Liolitsa D, Deliveliotis C. Manipulation of the nerve growth factor network in prostate cancer. Expert Opin Investig Drugs 2007;16:303-9. [Crossref] [PubMed]
- Desmet CJ, Peeper DS. The neurotrophic receptor TrkB: a drug target in anti-cancer therapy? Cell Mol Life Sci 2006;63:755-9. [Crossref] [PubMed]
- Adriaenssens E, Vanhecke E, Saule P, et al. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res 2008;68:346-51. [Crossref] [PubMed]
- Prueitt RL, Yi M, Hudson RS, et al. Expression of microRNAs and protein-coding genes associated with perineural invasion in prostate cancer. Prostate 2008;68:1152-64. [Crossref] [PubMed]
- Darios ES, Seitz B, Watts SW. Smooth muscle pharmacology in the isolated virgin and pregnant rat uterus and cervix. J Pharmacol Exp Ther 2012;341:587-96. [Crossref] [PubMed]
- Cole SW, Nagaraja AS, Lutgendorf SK, et al. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 2015;15:563-72. [Crossref] [PubMed]
- Entschladen F, Palm D, Niggemann B, et al. The cancer's nervous tooth: Considering the neuronal crosstalk within tumors. Semin Cancer Biol 2008;18:171-5. [Crossref] [PubMed]
- Lolas G, Bianchi A, Syrigos KN. Tumour-induced neoneurogenesis and perineural tumour growth:a mathematical approach. Sci Rep 2016;6:20684. [Crossref] [PubMed]
- Bunimovich YL, Keskinov AA, Shurin GV, et al. Schwann cells: a new player in the tumor microenvironment. Cancer Immunol Immunother 2017;66:959-68. [Crossref] [PubMed]
- Deborde S, Omelchenko T, Lyubchik A, et al. Schwann cells induce cancer cell dispersion and invasion. J Clin Invest 2016;126:1538-54. [Crossref] [PubMed]
- Demir IE, Boldis A, Pfitzinger PL, et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J Natl Cancer Inst 2014. [Crossref] [PubMed]
- Azam SH, Pecot CV. Cancer's got nerve: Schwann cells drive perineural invasion. J Clin Invest 2016;126:1242-4. [Crossref] [PubMed]
- Scanlon CS, Banerjee R, Inglehart RC, et al. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat Commun 2015;6:6885. [Crossref] [PubMed]
- Capek S, Howe BM, Amrami KK, et al. Perineural spread of pelvic malignancies to the lumbosacral plexus and beyond: clinical and imaging patterns. Neurosurg Focus 2015;39:E14. [Crossref] [PubMed]
- Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science 2013;341:1236361. [Crossref] [PubMed]
- Ali A, Pisipati S, Tewari A. Words of wisdom: Re: Autonomic nerve development contributes to prostate cancer progression. Eur Urol 2014;65:665-6. [Crossref] [PubMed]
- Zhao CM, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med 2014;6:250ra115. [Crossref] [PubMed]
- Ozaki H, Hiraoka T, Mizumoto R, et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today 1999;29:16-22. [Crossref] [PubMed]
- Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg 2004;240:260-8. [Crossref] [PubMed]
- Beard CJ, Chen MH, Cote K, et al. Perineural invasion is associated with increased relapse after external beam radiotherapy for men with low-risk prostate cancer and may be a marker for occult, high-grade cancer. Int J Radiat Oncol Biol Phys 2004;58:19-24. [Crossref] [PubMed]
- Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg 1996;223:384-94. [Crossref] [PubMed]
- Duraker N, Sisman S, Can G. The significance of perineural invasion as a prognostic factor in patients with gastric carcinoma. Surg Today 2003;33:95-100. [Crossref] [PubMed]
- Sevin BU, Lu Y, Bloch DA, et al. Surgically defined prognostic parameters in patients with early cervical carcinoma. A multivariate survival tree analysis. Cancer 1996;78:1438-46. [Crossref] [PubMed]
- Buda A, Fossati R, Colombo N, et al. Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the SNAP01 (Studio Neo-Adjuvante Portio) Italian Collaborative Study. J Clin Oncol 2005;23:4137-45. [Crossref] [PubMed]
- Vizza E, Pellegrino A, Milani R, et al. Total laparoscopic radical hysterectomy and pelvic lymphadenectomy in locally advanced stage IB2-IIB cervical cancer patients after neoadjuvant chemotherapy. Eur J Surg Oncol 2011;37:364-9. [Crossref] [PubMed]
- Lahousen M, Haas J, Pickel H, et al. Chemotherapy versus radiotherapy versus observation for high-risk cervical carcinoma after radical hysterectomy: A randomized, prospective, multicenter trial. Gynecol Oncol 1999;73:196-201. [Crossref] [PubMed]
- Rose PG. Advances in the management of cervical cancer. J Reprod Med 2000;45:971-8. [PubMed]
- Delgado G, Bundy B, Zaino R, et al. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol 1990;38:352-7. [Crossref] [PubMed]
- Ho CM, Chien TY, Huang SH, et al. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol Oncol 2004;93:458-64. [Crossref] [PubMed]
- Morice P, Castaigne D, Pautier P, et al. Interest of pelvic and paraaortic lymphadenectomy in patients with stage IB and II cervical carcinoma. Gynecol Oncol 1999;73:106-10. [Crossref] [PubMed]
- Pieterse QD, Kenter GG, Eilers PH, et al. An individual prediction of the future (disease-free) survival of patients with a history of early-stage cervical cancer, multistate model. Int J Gynecol Cancer 2008;18:432-8. [Crossref] [PubMed]
- Sartori E, Tisi G, Chiudinelli F, et al. Early stage cervical cancer: adjuvant treatment in negative lymph node cases. Gynecol Oncol 2007;107:S170-4. [Crossref] [PubMed]
- Singh N, Arif S. Histopathologic parameters of prognosis in cervical cancer--a review. Int J Gynecol Cancer 2004;14:741-50. [PubMed]
- Takeda N, Sakuragi N, Takeda M, et al. Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstet Gynecol Scand 2002;81:1144-51. [Crossref] [PubMed]
- Trimbos JB, Lambeek AF, Peters AA, et al. Prognostic difference of surgical treatment of exophytic versus barrel-shaped bulky cervical cancer. Gynecol Oncol 2004;95:77-81. [Crossref] [PubMed]
- Memarzadeh S, Natarajan S, Dandade DP, et al. Lymphovascular and perineural invasion in the parametria: a prognostic factor for early-stage cervical cancer. Obstet Gynecol 2003;102:612-9. [PubMed]
- Ozan H, Ozuysal S, Ediz B. Perineural invasion in early-stage cervical carcinoma. Eur J Gynaecol Oncol 2009;30:379-83. [PubMed]
- Tavares MB, Sousa RB, Oliveira e Silva T, et al. Prevalence of prognostic factors for cancer of the uterine cervix after radical hysterectomy. Sao Paulo Med J 2009;127:145-9. [Crossref] [PubMed]
- Horn LC, Meinel A, Fischer U, et al. Perineural invasion in carcinoma of the cervix uteri--prognostic impact. J Cancer Res Clin Oncol 2010;136:1557-62. [Crossref] [PubMed]
- Elsahwi KS, Barber E, Illuzzi J, et al. The significance of perineural invasion in early-stage cervical cancer. Gynecol Oncol 2011;123:561-4. [Crossref] [PubMed]
- Cho HC, Kim H, Cho HY, et al. Prognostic significance of perineural invasion in cervical cancer. Int J Gynecol Pathol 2013;32:228-33. [Crossref] [PubMed]
- Zhu Y, Zhang G, Yang Y, et al. Perineural invasion in early-stage cervical cancer and its relevance following surgery. Oncol Lett 2018;15:6555-61. [PubMed]
- Skręt-Magierło JE, Soja PJ, Skręt A, et al. Perineural space invasion in cervical cancer (FIGO IB1-IIB) accompanied by high-risk factors for recurrence. J Cancer Res Ther 2014;10:957-61. [Crossref] [PubMed]
- Cui L, Shi Y, Zhang GN. Perineural invasion as a prognostic factor for cervical cancer: a systematic review and meta-analysis. Arch Gynecol Obstet 2015;292:13-9. [Crossref] [PubMed]
- Aoun F, van Velthoven R. Lower urinary tract dysfunction after nerve-sparing radical hysterectomy. Int Urogynecol J 2015;26:947-57. [Crossref] [PubMed]
- Basaran D, Dusek L, Majek O, et al. Oncological outcomes of nerve-sparing radical hysterectomy for cervical cancer: a systematic review. Ann Surg Oncol 2015;22:3033-40. [Crossref] [PubMed]
- Chatterjee D, Katz MH, Rashid A, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol 2012;36:409-17. [Crossref] [PubMed]
- Cheng L, Slezak J, Bergstralh EJ, et al. Preoperative prediction of surgical margin status in patients with prostate cancer treated by radical prostatectomy. J Clin Oncol 2000;18:2862-8. [Crossref] [PubMed]
- Rigor BM Sr. Pelvic cancer pain. J Surg Oncol 2000;75:280-300. [Crossref] [PubMed]
- Katz B, Srougi M, Dall'Oglio M, et al. Perineural invasion detection in prostate biopsy is related to recurrence-free survival in patients submitted to radical prostatectomy. Urol Oncol 2013;31:175-9. [Crossref] [PubMed]
- Binmadi NO, Basile JR. Perineural invasion in oral squamous cell carcinoma: a discussion of significance and review of the literature. Oral Oncol 2011;47:1005-10. [Crossref] [PubMed]
- Penn R, Abemayor E, Nabili V, et al. Perineural invasion detected by high-field 3.0-T magnetic resonance imaging. Am J Otolaryngol 2010;31:482-4. [Crossref] [PubMed]
- Gil Z, Kelly KJ, Brader P, et al. Utility of a herpes oncolytic virus for the detection of neural invasion by cancer. Neoplasia 2008;10:347-53. [Crossref] [PubMed]
- Reade CJ, Eiriksson LR, Covens A. Surgery for early stage cervical cancer: how radical should it be? Gynecol Oncol 2013;131:222-30. [Crossref] [PubMed]
- Füle T, Máthé M, Suba Z, et al. The presence of human papillomavirus 16 in neural structures and vascular endothelial cells. Virology 2006;348:289-96. [Crossref] [PubMed]
- Isobe T, Takahashi K, Okuyama T. S100a0 (alpha alpha) protein is present in neurons of the central and peripheral nervous system. J Neurochem 1984;43:1494-6. [Crossref] [PubMed]
- Kato K, Suzuka K, Osaki T, et al. Unilateral or bilateral nerve-sparing radical hysterectomy: a surgical technique to preserve the pelvic autonomic nerves while increasing radicality. Int J Gynecol Cancer 2007;17:1172-8. [Crossref] [PubMed]


