
A randomized multicenter phase II trial of mecapegfilgrastim single administration versus granulocyte colony-stimulating growth factor on treating chemotherapy-induced neutropenia in breast cancer patients
Introduction
Breast cancer is considered as the most prevalent malignancy as well as the leading cause of cancer deaths in females with more than 2 million new breast cancer cases and 0.6 million breast cancer-induced deaths occurring in 2018 based on the estimation of the Global Cancer Statistics (1). Although great improvement in endocrine therapy, targeted therapy and immune therapy has been achieved and notably reduces mortality, chemotherapy is still the current key foundation treatment for breast cancer, which reduces the disease stage before surgery, enhances surgical resection, eliminates tumor residuals after surgery and improves overall survival in breast cancer patients (2,3). However, the majority of breast cancer patients received chemotherapy have been reported to develop neutropenia, which is a severe adverse event attributing to the myelosuppressive nature of chemotherapy regimens (4). And neutropenia impairs treatment efficacy, elevates risk of infection and increases length of hospitalization that often causes fatal consequences (4-6). Therefore, it is necessary to explore therapeutic drugs that treat or even prevent chemotherapy-induced neutropenia and improve prognosis in breast cancer patients.
Granulocyte colony-stimulating growth factor (G-CSF) is a hematopoietic growth factor that stimulates white blood cells proliferation, differentiation and survival, which could facilitate the recovery of neutropenia in cancer patients (7). As an analog of human G-CSF, filgrastim (with the brand name Neupogen) is the first approved drug for chemotherapy-induced neutropenia in cancer patients, while its short human half-life results in daily administration intravenously or subcutaneously, causing unnecessary suffering for patients and increase the risk of infection following therapeutic injection, which greatly limits its application in clinical settings (8). In order to solve this problem, pegfilgrastim, a pegylated form of filgrastim, has been invented with the human half-life being 15–80 hours and achieves a lower frequency of drug administration (once a chemotherapy cycle) as well as increases the drug bioavailability (9,10). Accumulating clinical researches have illustrated that pegfilgrastim presents better efficacy and similar safety profiles with filgrastim, and its clinical application is extensively promoted with good patient compliance in cancer patients (11,12). Although there is the unique superiority of pegfilgrastim for reducing neutropenia incidence and severity in chemotherapy-treated cancer patients, it has not been marketed in China yet.
Mecapegfilgrastim (HHPG-19K), a biosimilar of pegfilgrastim, is developed through cross linking a 19K polyethylene glycol with the N terminus of filgrastim by Jiangsu Hengrui Medicine Co. Ltd. (13,14). The pre-clinical data have demonstrated the analogical pharmacokinetics of HHFG-19K with pegfilgrastim, and a previous phase Ib clinical trial reports satisfactory tolerance as well as no resistance to HHPG-19K in non-small cell lung cancer (NSCLC) patients at a dosage of 60–200 µg/kg (13). In addition, the efficacy and safety of HHPG-19K as prophylaxis for chemotherapy-induced neutropenia in NSCLC patients have been shown to be comparable with G-CSF by a phase III study, whereas therapeutic effect of HHPG-19K in breast cancer patients is still not investigated (14). Considering the deficiency in long-acting anti-neutropenia drug in China and the limited information about HHFG-19K in breast cancer patients, this phase II clinical study was determined to evaluate the efficacy as well as safety of HHPG-19K, and investigate the optimized drug dosage for treating chemotherapy-induced neutropenia in breast cancer patients. This trial was approved by the State Food and Drug Administration of China (registration number: 2010L00501).
Methods
Study design
This study was a multi-center, randomized, active-controlled, phase II study, and a total of 182 breast cancer patients were enrolled from 20 medical centers (shown in Table S1). After enrollment, patients received first cycle of AT (epirubicin + docetaxel) or AC (epirubicin + cyclophosphamide) chemotherapy, during which, patients without chemotherapy-induced neutropenia were removed from following study, and those who had chemotherapy-induced neutropenia were further included in the following study. Subsequently, those patients who were eligible for following study were randomly given normal dose (100 µg/kg) or high dose (150 µg/kg) of HHPG-19K by single administration or daily injections of 5 µg/kg G-CSF at day 3 of second cycle of AT or AC chemotherapy. Then the efficacy and safety of HHPG-19K on treating chemotherapy-induced neutropenia were compared among HHPG-19K normal-dose group, HHPG-19K high-dose group and G-CSF group.
Full table
Patients
Between March 2011 and January 2012, 182 breast cancer patients about to receive AT or AC regimen for chemotherapy were recruited from 20 medical centers. The inclusion criteria were as follows: (I) diagnosed as breast cancer confirmed by histopathology examination; (II) aged 18–70 years old; (III) about to receive at least 2 cycles of AT regimen for neoadjuvant chemotherapy or 2 cycles of AC regimen for adjuvant chemotherapy; (IV) Eastern Cooperative Oncology Group (ECOG) performance score ≤1; (V) the hematopoietic function of bone marrow was normal, which was defined as white blood cell count (WBC) >4.0×109/L, absolute neutrophil count (ANC) >2.0×109/L and platelet count (PLT) >100×109/L; (VI) no obvious cardiac dysfunction; (VII) serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤1.5 times of upper limits of normal (ULN), serum total bilirubin (TBIL) ≤1.5 times of ULN and serum creatinine (Scr) ≤1.5 times of ULN; (VIII) able to comply with the study protocol (judged by researchers); (IX) volunteered to participate in this clinical trial, and able to understand the research process and sign the written informed consent. The exclusion criteria included: (I) previous history of bone marrow transplantation or stem cell transplantation; (II) complicated with acute infection and received systemic anti-infection treatment within 72 hours before chemotherapy; (III) had previously received systemic chemotherapy within 4 weeks; (IV) complicated with hematological diseases affecting hematopoietic function of bone marrow; (V) with serious accompanying disease affecting the safety and compliance of the trial, which were judged by researchers; (VI) women in gestation or lactation period; (VII) participated in other drug clinical trial 1 month before enrollment of this study; (VIII) previously received peg--recombinant human G-CSF (PEG-rhG-CSF) treatment; (IX) allergic or intolerable to the study drugs, rhG-CSF or other biologicals; (X) drug addicts.
Ethics statement
This study was approved by the Ethics Committee of the medical center (2011-02-18), and the protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients or their guardians signed the informed consents before enrollment.
Randomization
After first cycle of chemotherapy, patients who met the inclusion criteria of subsequent study were randomly assigned to three groups as a 1:1:1 ratio: HHPG-19K normal-dose (HHPG-19K-N) group (n=60), HHPG-19K high-dose (HHPG-19K-H) group (n=61) or G-CSF group (n=61). Based on the principle of minimum randomization, central randomization grouping was performed by use of the centralized-randomized grouping system provided by the Fourth Military Medical University. When an eligible patient was screened out, the researcher entered the random system to fill in the screening data and acquire the random number and the corresponding study drug, then the patient was treated with the system assigned drug. During central randomization process, factors including age, ECOG and ANC inhibition status in the first cycle of chemotherapy were considered as controlled factors to ensure equilibrium among groups.
Treatment protocols
- First cycle of chemotherapy: after baseline screening, all enrolled patients were treated with first cycle (cycle 1) of AT for neoadjuvant chemotherapy or AC regimen for adjuvant chemotherapy, which were administrated as follows: (i) AT regimen: epirubicin (Zhejiang Hisun Pharmaceutical Co., Ltd., Taizhou, Zhejiang, China) 75 mg/m2, intravenous drip, on day 1; docetaxel (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu, China) 75 mg/m2, intravenous drip, on day 1; (ii) AC regimen: epirubicin 100 mg/m2, intravenous drip, on day 1; cyclophosphamide (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu, China) 600 mg/m2, intravenous drip, on day 1. Both AT and AC regimens were repeated every 21 days for each cycle. Before the cycle 1, patients were not given prophylaxis drugs for chemotherapy-induced neutropenia. During the cycle 1, once the following conditions occurred, antibiotics or platelet transfusion were given to the patients: (i) the grade-4 neutropenia, (ii) febrile neutropenia (FN), (iii) clear infection, (iv) body temperature ≥38.5 °C with suspicious infectious fever, (v) grade-4 thrombocytopenia.
- Second cycle of chemotherapy: after the cycle 1, patients were excluded from following study if they did not have neutropenia during cycle 1. Furthermore, for consideration of patient safety, patients with following conditions during cycle 1 were also excluded from subsequent study: (i) grade-4 thrombocytopenia; (ii) grade-4 hemoglobin decline; (iii) severe cardiac toxicity as well as other status that were not suitable for subsequent study judged by researchers. And patients meeting the following criteria were included in subsequent study: (i) with the occurrence of neutropenia during cycle 1; (ii) with ANC ≥2.0×109/L and PLT ≥80×109/L before initiation of second cycle of chemotherapy. As for patients who were eligible for subsequent study, they received second cycle (cycle 2) of AT or AC chemotherapy, and the AT or AC regimen was given on day 1 of cycle 2. Dosage of chemotherapy drugs in cycle 2 among three groups was similar.
- Treatment for chemotherapy-Induced neutropenia: on the day 3 of cycle 2, patients in HHPG-19K-N group were treated with HHPG-19K (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu, China) 100 µg/kg by single subcutaneous injection; patients in HHPG-19K-H group were treated with HHPG-19K 150 µg/kg by single subcutaneous injection; as for patients in G-CSF group, they were treated with G-CSF (Jiangsu Wuzhong Pharmaceutical Group Co., Ltd., Suzhou, Jiangsu, China) 5 µg/kg by subcutaneous injection, then daily administration, and it was stopped if any of following drug withdrawal indications occurred: (i) continuous injection for 14 days; (ii) ANC ≥5.0×109/L in two consecutive examinations after ANC reached the lowest point; (3) ANC ≥15×109/L.
- Rescue application of G-CSF: if there were following situations during study, rescue application of 5 µg/kg G-CSF was allowed until ANC ≥5.0×109/L: (i) grade-4 neutropenia (ANC <0.5×109/L) or/and FN occurred in the cycle 1; (ii) during the observation period (use of HHPG-19K or G-CSF was stopped) of cycle 2, ANC <0.5×109/L or/and FN occurred more than 3 days. In addition to the above conditions, G-CSF, radiotherapy or other treatments affecting hemogram was not used in the chemotherapy cycle.
Data collection at baseline and blood monitoring during cycle 1 and cycle 2
After enrollment, patients’ baseline characteristics were collected, such as age, height, body weight, body surface areas, ECOG score, comorbidities, treatment history, planned chemotherapy regimen, level of ANC and so on. During cycle 1 and cycle 2, blood routine examination was performed daily from the first day of chemotherapy, and ANC profiles were recorded in detail.
Efficacy assessment
The primary efficacy endpoint was incidence of grade ≥3 neutropenia during cycle 2. And the second efficacy endpoints included incidence of grade 4 neutropenia during cycle 2, duration of grade ≥3 neutropenia during cycle 2, duration of grade 4 neutropenia during cycle 2, incidence of FN during cycle 2, level of ANC at different time point during cycle 2 and rescue application of G-CSF during cycle 2 and time to ANC recovery during cycle 2.
Safety assessment
Any adverse event (AE) occurred during study period was recorded in detail, which included description of events and all related symptoms, occurrence time, severity, duration, measures taken and outcomes. Grading of AE was assessed referring to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0, and whether the AEs were related to the study drugs was judged by researchers.
Definitions
Neutropenia was defined as ANC <2.0×109/L; grade ≥3 neutropenia was defined as ANC <1.0×109/L; grade 4 neutropenia was defined as ANC <0.5×109/L; time to ANC recovery was defined as the duration of ANC from nadir to more than 2.0×109/L. FN was defined as body temperature ≥38.5 °C concurrent with ANC <1.0×109/L. Serious AE was defined as the events that required hospitalization, prolonged hospitalization time, caused disability, affected work ability, endangered life or death or lead to congenital malformations during clinical trials.
Statistical analysis
The required sample size for this study was estimated based on previous study (14). Using an 80% power to detect a difference of 35% in the incidence of grade ≥3 neutropenia during cycle 2 between HHPG-19K groups and G-CSF group, with a two-sided 5% level of significance (α), required a sample size of 54 participants in each group. Accounting for loss follow up of up to 10%, 60 participants were required in each group, with a total sample size of 180. Since the number of cases was not capped in the design of the random grouping system, when the enrollment of 180 eligible subjects completed, the system was still accessible, resulting in a total of 182 patients included in current study. All analyses were performed in the full analysis set (FAS) and per-protocol population set (PPS). Due to the same conclusions between FAS analyses and PPS analyses, only the results of FAS (n=181) were disclosed in this present study. For the patients with missing data of efficacy in the FAS, last observation carried forward (LOCF) method was used for processing data. SPSS 22.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis and GraphPad Prism 7.01 software (GraphPad Software Inc., San Diego, CA, USA) was used for chart making. Count data were expressed as count (percentage) or 95% confidence interval, comparison among groups was determined by Chi-square test, fisher’s exact test or Kruskal-Wallis H test; continuous data were described as mean ± standard deviation with maximum and minimum values, and comparison among groups was determined by one-way ANOVA followed by post-hoc tests (Tukey’s). Kaplan-Meier curve and log-rank test were used to determine the difference in time to ANC recovery among groups. Reported statistical significance levels were all two-sided, and P value <0.05 was considered significant.
Results
Study flow
A total of 182 breast cancer patients about to receive at least 2 cycles of AT or AC neoadjuvant or adjuvant chemotherapy were initially enrolled in this trial and underwent cycle 1 chemotherapy (Figure 1). All of the 182 patients developed neutropenia after cycle 1 chemotherapy and were randomly assigned to HHPG-19K-N group (n=60), HHPG-19K-H group (n=61) and G-CSF group (n=61) as 1:1:1 ratio. (I) In HHPG-19K-N group, 60 patients received cycle 2 chemotherapy and 100 µg/kg of HHPG-19K was given on day 3; 59 patients in HHPG-19K-N group completed study were included in Per Protocol Set (PPS) (1 patient had poor compliance due to adverse events), and 60 patients was included in Full Analysis Set (FAS). (II) In HHPG-19K-H group, 61 patients received cycle 2 chemotherapy and 150 µg/kg of HHPG-19K was given on day 3; 56 patients in HHPG-19K-H group completed study and were included in PPS [but 5 were excluded for the reasons that: ALT violated the protocol requirement (n=2); violated the inclusion criteria before cycle 2 chemotherapy (n=1); lost follow up (n=1); others (n=1)], and 61 patients were included in FAS. (III) As for G-CSF group, 60 patients received cycle 2chemotherapy but 1 patient withdrew, and 5 µg/kg of G-CSF was given on day 3 of cycle 2, then daily; 56 patients in G-CSF group completed study were included in PPS [4 patients were excluded due to that: chemotherapy dose violated the protocol (n=2); violated the therapeutic regimen of G-CSF (n=1); others (n=1)], and 60 patients were included in FAS.
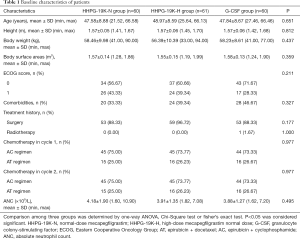
Baseline characteristics of breast cancer patients in HHPG-19K-N, HHPG-19K-H and G-CSF groups were listed in Table 1. Three-group comparison analysis exhibited that there was no difference regarding to age, height, body weight, body surface areas, ECOG score, comorbidities, treatment history, chemotherapy regimens in cycle 1, chemotherapy regimens in cycle 2 or ANC among 3 groups (all P>0.05).
Full table
ANC profiles during cycle 2 chemotherapy
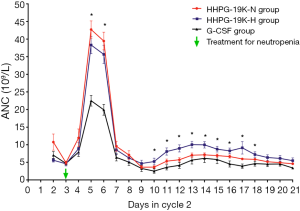
Detailed ANC profiles during cycle 2 were recorded, which showed that the mean values of ANC level in HHPG-19K-N, HHPG-19K-H and G-CSF groups peaked at day 5 and declined sharply from day 6 to day 9, and then it started to rise slightly until a second drop occurred at day 14 (Figure 2). The three-group comparison analysis revealed that the ANC levels differed at day 5, day 6 and from day 10 to day 18 among the 3 groups (all P<0.05). The two-group comparison analyses were then performed and disclosed that the ANC levels in both HHPG-19K-N and HHPG-19K-H groups were higher compared with G-CSF group at day 5 and day 6 (all P<0.001), and was elevated in HHPG-19K-H group compared to G-CSF group from day 10 to day 18 (all P<0.05). No difference in ANC levels between HHPG-19K-N and HHPG-19K-H groups at any time points during cycle 2 chemotherapy was observed (all P>0.05).
Incidence of grade ≥3 neutropenia during cycle 2 chemotherapy
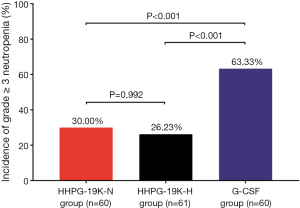
There were 30.00%, 26.23% and 63.33% of patients developing grade ≥3 neutropenia in HHPG-19K-N group, HHPG-19K-H group and G-CSF group, respectively (Figure 3). Three-group comparison analysis disclosed that the incidences of grade ≥3 neutropenia were different among the 3 groups (P<0.001), and the following two-group comparison analyses revealed that the incidence of grade ≥3 neutropenia was lower in both HHPG-19K-N (P<0.001) and HHPG-19K-H (P<0.001) groups compared with G-CSF group, while no difference was observed between HHPG-19K-N group and HHPG-19K-H group (P=0.992).
Incidence of grade 4 neutropenia, FN and grade ≥3/grade 4 neutropenia duration during cycle 2 chemotherapy
(I) Grade 4 neutropenia: the three-group comparison analysis exhibited that incidences of grade 4 neutropenia in HHPG-19K-N (6.67%), HHPG-19K-H (14.75%) and G-CSF groups (33.33%) were different (P=0.003) (Table 2). And the following two-group comparison analyses exhibited that the incidence of grade 4 neutropenia was lower in HHPG-19K-N (P<0.001) and HHPG-19K-H (P=0.019) groups compared with G-CSF group, while was similar between HHPG-19K-N and HHPG-19K-H groups (P=0.094). (II) FN: the three-group comparison analysis displayed that the incidences of FN were similar among HHPG-19K-N (0.00%), HHPG-19K-H (1.64%) and G-CSF groups (1.67%) (P=0.472). (III) Grade ≥3 neutropenia duration: the three-group comparison analysis exhibited that mean durations of grade ≥3 neutropenia among HHPG-19K-N (0.77±1.35 days), HHPG-19K-H (0.67±1.31 days) and G-CSF groups (3.30±3.37 days) varied (P<0.001), and the following two-group comparison analyses disclosed shorter mean duration of grade ≥3 neutropenia in HHPG-19K-N (P<0.001) and HHPG-19K-H (P<0.001) groups compared with G-CSF group, whereas no difference in mean duration of grade ≥3 neutropenia was observed between HHPG-19K-N and HHPG-19K-H groups (P=0.967). (IV) Grade 4 neutropenia duration: the three-group comparison analysis showed that grade 4 neutropenia duration among HHPG-19K-N (0.10±0.40 days), HHPG-19K-H (0.25±0.65 days) and G-CSF groups (0.97±1.80 days) were different (P<0.001), and the following two-group comparison analysis presented that the mean duration of grade 4 neutropenia was shorter in HHPG-19K-N (P<0.001) and HHPG-19K-H (P=0.002) groups compared with G-CSF group, while it was similar between HHPG-19K-N and HHPG-19K-H groups (P=0.745).

Full table
Rescue application of G-CSF during cycle 2 chemotherapy
According to the three-group comparison analysis, the numbers of patients received rescue application of G-CSF among HHPG-19K-N [0 (0.00%)], HHPG-19K-H [2 (3.28%)] and G-CSF groups [14 (23.33%)] were different (P<0.001) (Table 3). The following two-group comparison analyses displayed that the number of patients received rescue application of G-CSF was smaller in HHPG-19K-N (P<0.001) and HHPG-19K-H (P<0.001) groups compared with G-CSF group, Besides, the durations of G-CSF application between HHPG-19K-H group and G-CSF group were similar (P=0.187).

Full table
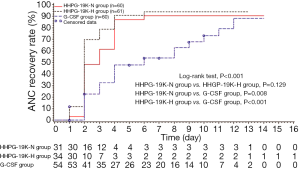
Time to ANC recovery during cycle 2 chemotherapy
Time to ANC recovery in HHPG-19K-N, HHPG-19K-H and G-CSF groups were illustrated in Figure 4. Three-group comparison analysis showed that time to ANC recovery among the 3 groups were different (P<0.001), and the following two-group comparison analyses revealed that the time to ANC recovery in HHPG-19K-N (P=0.008) and HHPG-19K-H groups (P<0.001) were shorter compared to G-CSF group, whereas no difference was observed between HHPG-19K-N and HHPG-19K-H groups (P=0.129).
Safety assessment
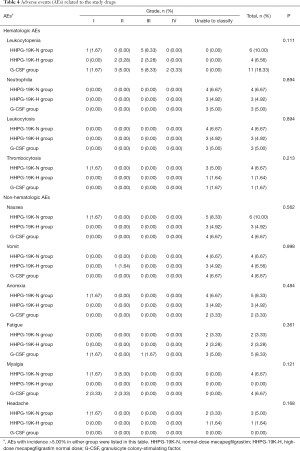
Hematologic and non-hematologic adverse events related to the study drug during cycle 2 were recorded, and the incidences of these adverse events were compared among HHPG-19K-N, HHPG-19K-H and G-CSF groups (Table 4). Three-group comparison analysis reported that there was no difference in incidences of hematologic adverse events including leukocytopenia, neutrophilia, leukocytosis and thrombocytosis or non-hematologic adverse events including nausea, vomit, anorexia, fatigue, myalgia and headache among the 3 groups (all P>0.05). There was no reported severe adverse event in each group during cycle 2 chemotherapy.
Full table
Subgroup analysis
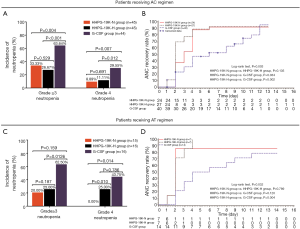
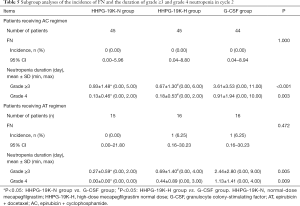
Taking into consideration that different chemotherapy regimens might have impact on efficacy outcomes, patients were further divided into subgroups depending on the chemotherapy regimen they received (AC or AT), and comparison of efficacy outcomes were carried out in each subgroup, which exhibited that HHPG-19K-N and HHPG-19K-H groups presented better primary and secondary endpoints compared with G-CSF group (Figure 5, Tables 5,6).
Full table

Full table
Discussion
In this randomized controlled trial, we discovered that in breast cancer patients: (I) HHPG-19K reduced the incidence of grade ≥3 neutropenia compared with G-CSF, and it also outperformed G-CSF with respect to secondary efficacy endpoints (including: grade 4 neutropenia, duration of grade ≥3 neutropenia, duration of grade 4 neutropenia, incidence of FN, rescue application of G-CSF and time to ANC recovery) independent of doses; (II) HHPG-19K was comparable with G-CSF regarding incidences of adverse events in treating chemotherapy-induced neutropenia; (III) there was no difference in efficacy and safety between HHPG-19K-N and HHPG-19K-H groups.
Neutropenia is a common complication of anthracycline or taxane-based chemotherapy due to myelosuppression, which limits the application of chemotherapy regimens and the treatment efficacy in non-myeloid malignancies including breast cancer (4-6,15). However, the management of neutropenia becomes more operative with the discovery of G-CSF, which increases the production of neutrophil precursors and differentiation of mature neutrophils (11). Although filgrastim (a human G-CSF analog) has been shown to reduce the incidence and duration of neutropenia and ameliorate the severity of myelosuppression, its short human half-life requires it to be administrated daily, which reduces patients’ compliance and limits its extensive clinical application (8). In this context, the long-acting pegfilgrastim is developed by covalently bonding a 20-kDa polyethylene glycol (PEG) molecule to the N-terminal methionine residue of filgrastim, and it is shown to have better efficacy, equal safety and more convenience compared to filgrastim (16-18). A multicenter, double-blind, placebo-controlled phase III study reports that single administration of pegfilgrastim greatly reduces the incidence of FN and FN-related hospitalization in docetaxel-treated breast cancer patients (19). Additionally, another study illuminates that pegfilgrastim is well tolerated in breast cancer patients due to the slow absorption properties, and it can be used as the therapeutic agent for severe neutropenia on outpatient’s basis (9). These clinical studies illustrate the advantages of pegfilgrastim in clinical application and equal safety compared to filgrastim. However, due to that pegfilgrastim is currently not marketed in China, Chinese cancer patients are still bearing the inconvenience of having to receive daily injection of G-CSF and the risk of other complications related to frequent drug administration. Therefore, the urgent demand of long-acting G-CSF needs to be met in order to alleviate patients distress from neutropenia.
Mecapegfilgrastim (HHPG-19K) is a biosimilar of pegfilgrastim developed in China, and has undergone pre-clinical study that reveal comparable pharmacokinetics and safety profiles with pegfilgrastim (13). There has been a previous study investigating the efficacy and safety of prophylactical use ofHHPG-19K in advanced NSCLC patients, which compares the efficacy endpoints and adverse events among 100 µg/kg HHPG-19K, fixed 6 mg HHPG-19K and G-CSF treatments, and reveals reduced incidence of grade ≥3 neutropenia, grade 4 neutropenia, shorter time to ANC recovery in both HHPG-19K groups compared with control group (14). Whereas for breast cancer patients, whose treatment relies greatly on chemotherapy and are more sensitive to chemotherapy regimens, the function of HHPG-19K is not yet investigated (19). By conducting this multi-center, randomized, phase II study, the efficacy of HHPG-19K on treating chemotherapy-induced neutropenia in breast cancer patients was evaluated. The results disclosed that both HHPG-19K-N group and HHPG-19K-H group had lower incidence of grade ≥3 neutropenia compared with G-CSF group, while no intergroup difference was observed between HHPG-19K-N group and HHPG-19K-H group. And similar trend was observed when comparing the secondary efficacy endpoints (including: grade 4 neutropenia, duration of grade ≥3 neutropenia, duration of grade 4 neutropenia, incidence of FN, rescue application of G-CSF and time to ANC recovery) across three groups. Although the patient types, study duration and chemotherapies in our study were different from the NSCLC study, both studies showed superiority of HHPG-19K in reducing incidence of grade ≥3, grade 4 neutropenia and time to ANC recovery compared with G-CSF, which indicated the clinical advantage of HHPG-19K over G-CSF (14). The possible explanations might be that: (I) as a biosimilar to pegfilgrastim, HHPG-19K was developed to stimulate the proliferation and maturation of neutrophil precursors and improve the function of mature neutrophils in cancer patients, which acts in the same manner as pegfilgrastim. And pegfilgrastim was reported to outperform G-CSF in clinical efficacies (13,14,20). Therefore, HHPG-19K resulted in superior efficacy endpoints in the present study as well; (II) due to longer human half-life of HHPG-19K, it was less frequently injected and resulted in lower risk of infection following therapeutic injection; hence it might have better patients’ compliance compared to G-CSF, and led to better clinical efficacy. It was also worth-noting that compared with clinical data of pegfilgrastim in breast cancer, the minimum value of ANC level was numerically higher in this study, which might be explained by that the anthracycline used in previous pegfilgrastim trials was doxorubicin, while in our study, epirubicin, which was relatively more myelosuppressive, was used (20,21). Furthermore, two-group comparison analysis displayed that the primary and secondary efficacy endpoints were similar between HHPG-19K-N and HHPG-19K-H group, suggesting that normal dose and high dose of HHPG-19K had equal treatment efficacy.
According to the previous clinical studies on pegfilgrastim in breast cancer patients, the safety indexes as well as drug tolerance of pegfilgrastim are similar to that of filgrastim (20-22). And for HHPG-19K, the previous phase III study of HHPG-19K in NSCLC patients reports that there is no difference in common adverse events including leukocytosis, fatigue, nausea, vomiting or hemoglobin decline among 100 µg/kg HHPG-19K, fixed 6 mg HHPG-19K and G-CSF treatments (14). In line with the above studies, we observed no difference in study drug-related adverse events among HHPG-19K-N, HHPG-19K-H and G-CSF groups. In addition, the types of adverse events induced by HHPG-19K were similar to that of pegfilgrastim, except for that pegfilgrastim led to mostly joint and back pain, while for HHPG-19K, the pain mainly manifested as myalgia (12,18); and the incidences of common adverse events caused by HHPG-19K were numerically lower compared with that caused by pegfilgrastim in breast cancer patients (20,21). These might be attributed to the varied chemotherapy regimens, patients’ disease stages and races selected in different studies. Besides, compared to the previous phase III study of HHPG-19K in NSCLC patients, the incidence of leukocytosis and fatigue in 100 µg/kg HHPG-19K group was lower in our study (6.67% vs. 8.51%; 3.33% vs. 4.26%), but nausea and vomiting rates were higher (10.00% vs. 2.13%; 6.67% vs. 0.00%) (14). This might be due to that the patient types (NSCLC vs. breast cancer) and the uses of HHPG-19K (prophylaxis vs. therapeutic) were different between the two studies. These safety data indicated that HHPG-19K is equally well-tolerated compared with G-CSF in breast cancer patients undergoing chemotherapy. Moreover, comparison of adverse events between HHPG-19K-N and HHPG-19K-H groups disclosed no difference, which indicated that both normal dose and high dose of HHPG-19Kwere well tolerated in breast cancer patients.
Furtherly, subgroup analyses were performed to eliminate the effect of chemotherapy regimens on study outcomes by dividing breast cancer patients into AC-treated and AT-treated patients. Comparisons of clinical efficacy endpoints across HHPG-19K-N, HHPG-19K-H and G-CSF groups displayed that in both AC and AT treated patients, HHPG-19K-N and HHPG-19K-H resulted in better primary and secondary efficacy endpoints compared with G-CSF group. These suggested that HHPG-19K was superior to G-CSF in terms of clinical efficacy regardless of the chemotherapy regimens that patients received.
There were still several limitations in our study. To begin with, time to ANC recovery rate was used as one of the secondary efficacy endpoints, while there were cases whose ANC did not recover throughout the whole chemotherapy cycle or decreased after recovery, which might impair the outcomes. Therefore, reduction duration of grade ≥3 and grade 4 neutropenia instead of time to ANC recovery could be recommended as assessment for efficacy in the future phase III study. In addition, this study was not a blinded study, thus, the subjective consciousness of patients and the physician might influence the clinical outcomes. The treatment efficacy and safety of HHPG-19Kas treatment for chemotherapy-induced neutropenia were evaluated in the present study, whereas its prophylactic use still needed to be investigated in further studies.
Conclusions
In conclusion, HHPG-19K presents with better clinical efficacy as well as equal tolerance compared with G-CSF in treating chemotherapy-induced neutropenia in breast cancer patients.
Acknowledgements
This study was funded by Jiangsu Hengrui Medicine Co. Ltd.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of the medical center (2011-02-18) and written informed consent was obtained from all patients.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Barbosa CRF, Falcone AB, Buzaid AC, et al. Neoadjuvant therapy for breast cancer treatment: an expert panel recommendation from the Brazilian Society of Breast Surgeons 2018. Breast Cancer Res Treat 2018;172:265-72. [Crossref] [PubMed]
- Tan W, Yang M, Yang H, et al. Predicting the response to neoadjuvant therapy for early-stage breast cancer: tumor-, blood-, and imaging-related biomarkers. Cancer Manag Res 2018;10:4333-47. [Crossref] [PubMed]
- Chan A, Chen C, Chiang J, et al. Incidence of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer 2012;20:1525-32. [Crossref] [PubMed]
- Kim HS, Lee SY, Kim JW, et al. Incidence and Predictors of Febrile Neutropenia among Early-Stage Breast Cancer Patients Receiving Anthracycline-Based Chemotherapy in Korea. Oncology 2016;91:274-82. [Crossref] [PubMed]
- Ma RM, Chen CZ, Zhang W, et al. Prognostic Value of Chemotherapy-Induced Neutropenia at the First Cycle in Invasive Breast Cancer. Medicine (Baltimore) 2016;95:e3240. [Crossref] [PubMed]
- Schroder CP, de Vries EG, Mulder NH, et al. Prevention of febrile leucopenia after chemotherapy in high-risk breast cancer patients: no significant difference between granulocyte-colony stimulating growth factor or ciprofloxacin plus amphotericin B. J Antimicrob Chemother 1999;43:741-3. [Crossref] [PubMed]
- Tan H, Tomic K, Hurley D, et al. Comparative effectiveness of colony-stimulating factors for febrile neutropenia: a retrospective study. Curr Med Res Opin 2011;27:79-86. [Crossref] [PubMed]
- Stathopoulos GP, Dimou E, Stathopoulos J, et al. Therapeutic administration of pegfilgrastim instead of prophylactic use. Anticancer Res 2005;25:2445-8. [PubMed]
- Di Lorenzo G, D'Aniello C, Buonerba C, et al. Peg-filgrastim and cabazitaxel in prostate cancer patients. Anticancer Drugs 2013;24:84-9. [Crossref] [PubMed]
- Morishita M, Leonard RC. Pegfilgrastim; a neutrophil mediated granulocyte colony stimulating factor-expanding uses in cancer chemotherapy. Expert Opin Biol Ther 2008;8:993-1001. [Crossref] [PubMed]
- Mitchell S, Li X, Woods M, et al. Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: A systematic review. J Oncol Pharm Pract 2016;22:702-16. [Crossref] [PubMed]
- Yan B, Zhang W, Lu F, et al. Safety of polyethylene glycol recombinant human granulocyte colony-stimulating factor in treating non-small cell lung cancer patients at I b stage. Asian Pac J Trop Med 2013;6:912-5. [Crossref] [PubMed]
- Zhou C, Huang Y, Wang D, et al. A Randomized Multicenter Phase III Study of Single Administration of Mecapegfilgrastim (HHPG-19K), a Pegfilgrastim Biosimilar, for Prophylaxis of Chemotherapy-Induced Neutropenia in Patients With Advanced Non-Small-Cell Lung Cancer (NSCLC). Clin Lung Cancer 2016;17:119-27. [Crossref] [PubMed]
- Aarts MJ, Grutters JP, Peters FP, et al. Cost effectiveness of primary pegfilgrastim prophylaxis in patients with breast cancer at risk of febrile neutropenia. J Clin Oncol 2013;31:4283-9. [Crossref] [PubMed]
- Vose JM, Crump M, Lazarus H, et al. Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J Clin Oncol 2003;21:514-9. [Crossref] [PubMed]
- Biganzoli L, Untch M, Skacel T, et al. Neulasta (pegfilgrastim): a once-per-cycle option for the management of chemotherapy-induced neutropenia. Semin Oncol 2004;31:27-34. [Crossref] [PubMed]
- Lambertini M, Ferreira AR, Del Mastro L, et al. Pegfilgrastim for the prevention of chemotherapy-induced febrile neutropenia in patients with solid tumors. Expert Opin Biol Ther 2015;15:1799-817. [Crossref] [PubMed]
- Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 2005;23:1178-84. [Crossref] [PubMed]
- Holmes FA, O'Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 2002;20:727-31. [Crossref] [PubMed]
- Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 2003;14:29-35. [Crossref] [PubMed]
- Zhang W, Jiang Z, Wang L, et al. An open-label, randomized, multicenter dose-finding study of once-per-cycle pegfilgrastim versus daily filgrastim in Chinese breast cancer patients receiving TAC chemotherapy. Med Oncol 2015;32:147. [Crossref] [PubMed]


