
Is waist circumference a negative predictor of calcaneal bone mineral density in adult Chinese men with normal weight?
Introduction
Osteoporosis is a metabolic skeletal disease characterized by low bone mass and microarchitectural deterioration, which easily leads to increase in bone fragility and susceptibility to fracture (1). Generally, bone mineral density (BMD) is used as a valid parameter to evaluate bone profile, and dual energy X-ray absorptiometry (DXA) is the gold standard for diagnosing osteoporosis and predicting risk of fracture (2). However, compared with DXA, the advantage of calcaneal quantitative ultrasound (QUS) method is cheaper, more portable and less exposed to X-ray. Thus, QUS is considered as a priority in large community-based epidemiological researches.
More recently, the relationship between osteoporosis and obesity, especially for central obesity, has become a new research hotspot. However, the conclusions remain controversial. Fu et al. reported that increased central body fat was negatively associated with BMD in Chinese women (3). On the contrary, a study performed in Taiwan came to an opposite conclusion (4). Waist circumference (WC) is regarded as the most reliable surrogate of central obesity (5). Increased WC has been demonstrated to be associated with higher mortality independently of body mass index (BMI) (6). Literature indicates that BMI is a well-described determinant for osteoporosis, and lower BMI is associated with lower BMD (7,8). Moderately increased BMI is thought to protect against osteoporosis (9). Previous researches on the association between abdominal obesity and BMD were completed in overweight or obese population as assessed by BMI (4,10,11), but it remains unclear about the effect of increased WC on BMD in the population with normal BMI (normal weight).
In addition, the lifestyle, diet habit and definition of obesity for Asian population (12) are different from Caucasian population, and more surveys on osteoporosis were performed in women than men. The aim of the present study was to explore the relationship between WC (quartiles) and BMD at the calcaneus in Chinese male adult population with normal-weight (BMI: 18.5–22.9 kg/m2).
Methods
Study design and population
This was a cross-sectional study. A total of 4,663 male participants aged 40 years or older residing in Ningde and Wuyishan, two cities locating in Fujian province of China, were randomly recruited between 2011 and 2012. Each should complete a questionnaire, undergo anthropometric and calcaneus QUS measurements and have blood sample taken. According to the definition of normal BMI for Asians by International Obesity Taskforce (13), the enrolled male residents with BMI ranging from 18.5 to 22.9 kg/m2 were included in the analysis. Participants were excluded if they lacked data for necessary items or had been taken the following drugs over the past 2 weeks: thiazide diuretics, glucocorticoids, thiazolidinediones, statin, fibrate, non-steroidal anti-inflammatory drugs, calcium or vitamin D supplement, bisphosphonate, calcitonin, thyroxine, anti-thyroid or any kind of diet pills. Based on this exclusion criteria, 1,583 men were included in the analysis. The study protocol was approved by the ethics committee of Fujian Provincial Hospital and written informed consent was obtained from each participant.
Data collection
Each subject was interviewed by a well-trained interviewer to complete a structured questionnaire. The content of questionnaire referred to personal history of diseases and medication, diet and lifestyle. Participants were asked to provide necessary information about drugs they had taken over the past 2 weeks. The consumption of smoke and alcohol is classified into three levels: current (current consumption over the past 6 months), ever (past consumption for more than 6 months) and never. We regard the first two levels as the positive one.
Anthropometric measurements
A general physical examination was performed for each participant. Height and weight were measured when participants were wearing light clothing and no shoes. Both were measured to the nearest 0.1 kg and 0.1 cm, respectively. BMI was calculated as body weight (kg) divided by height squared (m2). WC of each participant in a standing position was measured midway between the lateral lower rib margin and the superior anterior iliac crest. Blood pressure was measured by standard method.
Biochemical assay
A 75-g oral glucose tolerance test (OGTT) was administered after an overnight fast, and blood plasma was drawn at 0, 120 min for plasma glucose measurement. All serum lipids were determined in the fasting state. Fasting plasma glucose (FPG), 2-hour plasma glucose (2hPG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), total cholesterol (TC) were measured using chemiluminescence methods with an autoanalyzer. Fasting serum insulin concentrations were determined with the Phadebas Insulin Test (Pharmacia, Uppsala, Sweden) by a radioimmunosorbent technique.
Calcaneal QUS assessment
QUS measurements of the calcaneus were assessed using Achilles Express ultrasound (GE Lunar Corp., Madison, WI, USA) in Wuyishan and Sahara (Hologic, Waltham, MA, USA) in Ningde, respectively. Instrument output parameters include BMD (T-score), estimated BMD (eBMD) (g/cm2), broadband ultrasound attenuation (BUA) (dB/MHz) and speed of sound (SOS) (m/s). T-scores of BMD were calculated using the database of healthy Chinese youngsters in same study area as reference. The ultrasound parameters were measured twice on dominant calcaneus by a well-trained doctor and the mean value was used for analysis. Both QUS instruments were calibrated daily according to the manufacturer’s recommendations before measurement.
Diagnostic criteria
Hypertension is defined as systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg. Dyslipidemia was defined as self-reported current treatment with cholesterol-lowering medication or having 1 or more of the following: TC ≥5.17 mmol/L, TG ≥1.69 mmol/L, HDL-C ≤1.03 mmol/L, or LDL-C ≥3.38 mmol/L. Diabetes is diagnosed according to the American Diabetes Association (ADA) criteria (14): FPG ≥7.0 mmol/L or 2hPG ≥11.1 mmol/L. According to the World Health Organization (WHO) definition (15), osteoporosis is defined as a calcaneal BMD of >2.5 standard deviations (SDs) below the average peak BMD of young, healthy Chinese men in same study area (T-score <−2.5), and osteopenia is defined as a calcaneal BMD between 1 and 2.5 SDs below the peak BMD (−2.5< T-score <−1).
Statistical analysis
Variables are expressed as mean ± SD for continuous data or percentage for categorical variables. One-way ANOVA and non-parametric tests were used to compare the statistical difference between continuous variables, and the comparison of proportion was performed by chi-square test. WC was stratified by quartiles (Q1–Q4: <71, 71–75, 75–78, >78 cm). The first quartile was set as reference. Three multiple linear regression models were established to test the association between WC quartiles and calcaneal BMD. Model 1 was adjusted for age and BMI. Model 2 was further adjusted for smoke, drink and physical activity (PA). Lastly, covariates of diabetes mellitus (DM), dyslipidemia, hypertension, product of bean, milk and insulin were added into Model 3 on basis of Model 2 in order to investigate the effects of disease, diet and insulin on BMD. Adjusted for the same confounding factors, another logistic regression model was established to identify the risk of osteopenia and osteoporosis across WC quartiles. SPSS (version 19.0 for windows, SPSS Inc., Chicago, IL, USA) was used for statistical analysis. P<0.05 was considered significant (two-tailed).
Results
Descriptive statistics
As shown in Table 1, mean age of the whole population was 54.6±9.8 years. Anthropometric and biochemical parameters of participants in the present study were almost normal. The proportion of smokers was nearly 1.6 times as much as that of drinkers. PA was prevalent, and more than 60% of participants had ever taken part in activities.

Full table
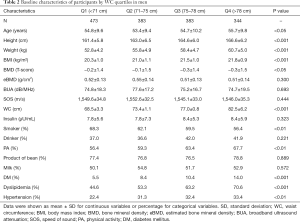
The baseline characteristics of men across WC quartiles were presented in Table 2. Compared with men in the lower quartile, those in the higher quartile were more likely to have significantly higher height, weight, BMI and higher prevalence of PA, DM, dyslipidemia and hypertension, but less likely to smoke. There were no significant differences in eBMD, BUA, SOS, serum insulin, drink, product of bean and milk across quartiles.
Full table
Multiple regression analysis
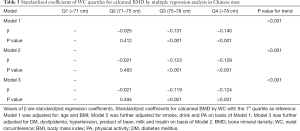
Standardized coefficients of WC for calcaneal BMD by multiple linear regression analysis were shown in Table 3. In Model 1, adjustment was made for age and BMI, there was a significant linear relationship between WC quartiles and calcaneal BMD except for quartile 2 (71–75 cm). BMD was negatively associated with WC. Model 2 was further adjusted for smoke, drink and PA on basis of Model 1. Standardized coefficients of Model 2 were attenuated slightly but remained significant. The same situation was also present in the Model 3 after adjusting for DM, dyslipidemia, hypertension, product of bean, milk and insulin. There was no significant difference between BMD and quartile 2 of WC in all three Models.
Full table
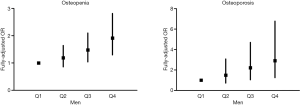
As shown in Figure 1, with Q1 as reference, the fully-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of osteopenia were 1.188 (0.858, 1.645) for Q2, 1.479 (1.041, 2.100) for Q3, and 1.913 (1.299, 2.816) for Q4, and corresponding results of osteoporosis were 1.493 (0.724, 3.078) for Q2, 2.214 (1.044, 4.695) for Q3, and 2.908 (1.247, 6.779) for Q4. Apparently, a linear trend was observed that higher fully-adjusted OR of osteopenia or osteoporosis was with higher WC quartile. After the same covariates as in the Model 3 of Table 3 were controlled, the statistical difference was only present in quartile 1, 3, and 4, but not in quartile 2 in both logistic regression models.
Discussion
In the present study, a significant relationship between WC quartiles and calcaneal BMD was present after adjusting for a series of covariates in adult men with normal weight. Our data indicated that, for the normal range of BMI, WC had a significant negative association with calcaneal BMD in adult Chinese men. Correspondingly, the risks of osteopenia and osteoporosis also correlated with WC quartiles positively.
Although several epidemiological researches (3,4,10,11) have documented that abdominal fat as measured by different anthropometric parameters is a strong predictor of bone mass, in this area, previous analyses focused more on obese or overweight population. Generally, a higher BMI or weight was thought to protect against bone loss by activating osteoblasts in response to mechanical load and muscle stress (9,16). In contrast, slender figures with low BMI were more likely to suffer osteoporosis and bone loss (17). Therefore, to explore the effect of abdominal fat on bone mass with minimal influence of BMI, a population with normal BMI was defined as the target population in our study. It is the first research to report the negative association between WC and BMD in Asian normal-weight males.
For researches about the effect of abdominal fat on bone, previously, most focused more on the population with whole spectrum of weight or female subjects only. A large cross-sectional survey conducted in Korean population (18), which included obese and overweight participants, demonstrated that WC was negatively correlated with BMD after adjusting for age and weight in both male and female subjects. Even WC was related to BMC independently of fat mass. Study on overweight and obese postmenopausal women in Turkey had conflicting conclusions in different sites, a significant positive association was observed between WC and total hip BMD, whereas WC was negatively associated with BMD in non-weight-bearing site, such as forearm (19). Likewise, the negative associations between abdominal fat and bone mass were also documented in other studies by alternatives instead of WC (3,10,11,20). However, results were also controversial. In a community-based study on elderly Taiwanese women, WC was correlated positively with lumbar, hip and femoral neck BMD (4). Another research focusing the relations of subcutaneous and visceral fat in healthy females also indicated that WC was positively related with bone (21). Compared with pre-existing researches, the current study was confined to the normal-weight adult Chinese males, which is expected to give some clues for their bone healthcare.
As previously reported, WC is frequently employed as a surrogate for abdominal adiposity and it contains abdominal subcutaneous and visceral fat depots (21,22). Increased WC means that abdominal fat including subcutaneous and visceral fat increases proportionally. Increment of abdominal adipose not only stimulates osteoblast differentiation by adding a mechanical load to bone, but also releases adipocyte-derived and pro-inflammatory cytokines to regulate bone metabolism. Several potential mechanisms probably account for our findings. Firstly, obesity and osteoporosis have been proven to share the same pathogenesis of chronic inflammation (23). As a marker of systemic inflammation, C-reactive protein (CRP) is elevated in abdominal obesity (24) and positively correlates with WC (25). Likewise, adipose tissue also produces other pro-inflammatory cytokines (26), such as tumor necrosis factor-α (TNF-α) and interlukin-6 (IL-6). These upregulated cytokines promote osteoporosis by stimulating osteoclast activity and bone resorption through the regulation of RANKL/RANK/OPG pathway (23,27). In addition, adipocyte and osteoblast arise from the same multipotential mesenchymal stem cell (28). The stem cell could differentiate into adipocyte or osteoblast equally. This balance is maintained by a series of common factors, such as PPARγ, Wnt, TGF-β, leptin, and estrogen (29). The imbalance between osteogenesis and adipogenesis is controlled by PPARγ (30), which predicts that increasing abdominal adipose may increase adipocyte differentiation and fat accumulation but decrease osteoblast differentiation and bone formation (osteoblastogenesis) (23). Thirdly, abdominal adipose tissue is also an organ to release the adipocyte-derived cytokines, especially for leptin and adiponectin. Leptin, which stimulates inflammatory and platelet response (31), has detrimental effect on bone in a mouse model (32). Adiponectin, another adipokine, inhibits osteoclastogenesis, reduces bone resorption, and increases bone mass (33). In contrast with leptin, adiponectin suppresses inflammatory responses by inhibiting TNF-α-induced NF-κB activation (34). Generally, it reduces in obese population. For the relation with abdominal adipose, Zhuo et al. reported that adiponectin was inversely correlated with WC in Chinese population (35), and another study also found that it was inversely associated with visceral fat (36). Fourthly, the effect of testosterone on bone metabolism should not be ignored. With age increasing, serum testosterone concentration declines in men (37), which is significantly associated with bone loss (38). Moreover, the reduced total and free testosterones were related with higher WC (39). It may suggest that WC should be an anthropometric measurement to predict endogenous testosterone levels preferably.
Only 27% and 38% of total body weight in white men and women is attributable to fat mass, respectively (40). Since abdominal fat is a part of total fat mass, that means the proportion of abdominal fat among body weight is small. Although increased body weight protects against bone loss, weight-associated gravitational forces due to increased fat mass may be insufficient to explain the impact of fat mass on bone. It is notable that positive relationship between body weight and bone mass is conditioned by body composition parameters (lean mass and fat mass). Lean mass quite matters in this relation instead of fat mass. For example, increase in lean mass is associated with higher bone mineral content at femoral neck in men, and significance disappears after adjusting for lean mass, indicating the principal role of lean mass in the maintenance of bone mass (41). In addition, correlation between lean mass and BMD is higher than that between fat mass and BMD in adult men (42). Note that, our focus is normal-weight Asian males, the positive effect of weight on BMD must be much less than overweight or obese population and is further statistically removed by regression model (Model 1 showed that result remained significant independently of age and BMI). Its beneficial effect on bone metabolism may be counteracted by the comprehensively detrimental effects of increased pro-inflammatory cytokines and leptin, reduced adipokines and declining age-dependent testosterone. However, we didn’t measure these cytokines or hormones in the current study. From this viewpoint, the raised cytokine-hormone hypothesis perhaps explains the results partially and remains to be verified by further investigations.
The strength of the present study is that the amount of sample was large. In this population-based research, a total of 1,583 male participants were included in the final analysis. Application of calcaneal QUS ensured that data about bone status could be obtained in the large community-based epidemiological survey. Additionally, we used WC as the predictor of abdominal fat to explore the association with BMD rather than others, because it was easy to be measured precisely and conveniently. More importantly, except for the generally accepted confounders, the other confounding factors including chronic diseases, diet and fasting insulin, which may disturb the valid relation of WC with BMD, were also controlled.
Our study had some limitations as well. Firstly, the research was performed in the adult Chinese male population with normal BMI. The result can’t be extrapolated to women or other ethnicities. Secondly, the cross-sectional study design limited our ability to prove causality. Thirdly, DXA as the gold standard for measuring BMD was not used in the present study, which may lower the precision of BMD measurement to some extent. The reason why we used QUS is because this is a community-based epidemiological survey and QUS is more convenient and suitable for us to do research than DXA. Fourthly, we didn’t determine the concentrations of CRP, pro-inflammatory cytokines, leptin, adiponectin and testosterone, further studies should be done to complete the measurements.
In conclusion, WC is a negative predictor of calcaneal BMD in the adult Chinese men with normal weight. Correspondingly, the risks of osteopenia and osteoporosis also increased across WC. It is the first time to investigate the relationship between WC and BMD in normal-weight Asian males. It is implicated that, even for the normal-weight adult men, caution of increased WC is still meaningful for the prevention of osteoporosis.
Acknowledgements
Funding: This study was supported by grants from the Chinese Medical Association Foundation and Chinese Endocrine Society (grant No. 12020240314).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the ethics committee of Fujian Provincial Hospital (No. 2011011) and written informed consent was obtained from each participant.
References
- Sambrook P, Cooper C. Osteoporosis. Lancet 2006;367:2010-8. [Crossref] [PubMed]
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 2011;377:1276-87. [Crossref] [PubMed]
- Fu X, Ma X, Lu H, et al. Associations of fat mass and fat distribution with bone mineral density in pre- and postmenopausal Chinese women. Osteoporos Int 2011;22:113-9. [Crossref] [PubMed]
- Chang CS, Chang YF, Wang MW, et al. Inverse relationship between central obesity and osteoporosis in osteoporotic drug naive elderly females: The Tianliao Old People (TOP) Study. J Clin Densitom 2013;16:204-11. [Crossref] [PubMed]
- Turcato E, Bosello O, Di Francesco V, et al. Waist circumference and abdominal sagittal diameter as surrogates of body fat distribution in the elderly: their relation with cardiovascular risk factors. Int J Obes Relat Metab Disord 2000;24:1005-10. [Crossref] [PubMed]
- Jacobs EJ, Newton CC, Wang Y, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med 2010;170:1293-301. [Crossref] [PubMed]
- Felson DT, Zhang Y, Hannan MT, et al. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 1993;8:567-73. [Crossref] [PubMed]
- Nguyen TV, Center JR, Eisman JA. Osteoporosis in elderly men and women: effects of dietary calcium, physical activity, and body mass index. J Bone Miner Res 2000;15:322-31. [Crossref] [PubMed]
- Wardlaw GM. Putting body weight and osteoporosis into perspective. Am J Clin Nutr 1996;63:433S-436S. [Crossref] [PubMed]
- Bhupathiraju SN, Dawson-Hughes B, Hannan MT, et al. Centrally located body fat is associated with lower bone mineral density in older Puerto Rican adults. Am J Clin Nutr 2011;94:1063-70. [Crossref] [PubMed]
- Katzmarzyk PT, Barreira TV, Harrington DM, et al. Relationship between abdominal fat and bone mineral density in white and African American adults. Bone 2012;50:576-9. [Crossref] [PubMed]
- Weisell RC. Body mass index as an indicator of obesity. Asia Pac J Clin Nutr 2002;11 Suppl 8:S681-4. [Crossref]
- World Health Organization, Regional Office for the Western Pacific; International Diabetes Institute; International Association for the Study of Obesity; International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000 Feb. Available online: http://iris.wpro.who.int/handle/10665.1/5379
- American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care 2012;35 Suppl 1:S11-63. [Crossref] [PubMed]
- Kanis JA, Melton LJ 3rd, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res 1994;9:1137-41. [Crossref] [PubMed]
- Hsu YH, Venners SA, Terwedow HA, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr 2006;83:146-54. [Crossref] [PubMed]
- Ravn P, Cizza G, Bjarnason NH, et al. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res 1999;14:1622-7. [Crossref] [PubMed]
- Kim JH, Choi HJ, Kim MJ, et al. Fat mass is negatively associated with bone mineral content in Koreans. Osteoporos Int 2012;23:2009-16. [Crossref] [PubMed]
- Agbaht K, Gurlek A, Karakaya J, et al. Circulating adiponectin represents a biomarker of the association between adiposity and bone mineral density. Endocrine 2009;35:371-9. [Crossref] [PubMed]
- Russell M, Mendes N, Miller KK, et al. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab 2010;95:1247-55. [Crossref] [PubMed]
- Gilsanz V, Chalfant J, Mo AO, et al. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 2009;94:3387-93. [Crossref] [PubMed]
- Ross R, Rissanen J, Hudson R. Sensitivity associated with the identification of visceral adipose tissue levels using waist circumference in men and women: effects of weight loss. Int J Obes Relat Metab Disord 1996;20:533-8. [PubMed]
- Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res 2011;6:30. [Crossref] [PubMed]
- Lapice E, Maione S, Patti L, et al. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care 2009;32:1734-6. [Crossref] [PubMed]
- Nakamura H, Ito H, Egami Y, et al. Waist circumference is the main determinant of elevated C-reactive protein in metabolic syndrome. Diabetes Res Clin Pract 2008;79:330-6. [Crossref] [PubMed]
- Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001;280:E745-51. [Crossref] [PubMed]
- Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology 2001;142:5050-5. [Crossref] [PubMed]
- Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med 2009;122:409-14. [Crossref] [PubMed]
- Zhao LJ, Jiang H, Papasian CJ, et al. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res 2008;23:17-29. [Crossref] [PubMed]
- Kang S, Bennett CN, Gerin I, et al. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem 2007;282:14515-24. [Crossref] [PubMed]
- Canavan B, Salem RO, Schurgin S, et al. Effects of physiological leptin administration on markers of inflammation, platelet activation, and platelet aggregation during caloric deprivation. J Clin Endocrinol Metab 2005;90:5779-85. [Crossref] [PubMed]
- Elefteriou F, Takeda S, Ebihara K, et al. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A 2004;101:3258-63. [Crossref] [PubMed]
- Oshima K, Nampei A, Matsuda M, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 2005;331:520-6. [Crossref] [PubMed]
- Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 2000;102:1296-301. [Crossref] [PubMed]
- Zhuo Q, Wang ZQ, Fu P, et al. Association between adiponectin and metabolic syndrome in older adults from major cities of China. Biomed Environ Sci 2010;23:53-61. [Crossref] [PubMed]
- Nomura K, Eto M, Kojima T, et al. Visceral fat accumulation and metabolic risk factor clustering in older adults. J Am Geriatr Soc 2010;58:1658-63. [Crossref] [PubMed]
- Snyder PJ. Effects of age on testicular function and consequences of testosterone treatment. J Clin Endocrinol Metab 2001;86:2369-72. [PubMed]
- Chin KY, Soelaiman IN, Naina Mohamed I, et al. Testosterone is associated with age-related changes in bone health status, muscle strength and body composition in men. Aging Male 2012;15:240-5. [Crossref] [PubMed]
- Svartberg J, von Muhlen D, Sundsfjord J, et al. Waist circumference and testosterone levels in community dwelling men. The Tromso study. Eur J Epidemiol 2004;19:657-63. [Crossref] [PubMed]
- Zhao LJ, Liu YJ, Liu PY, et al. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 2007;92:1640-6. [Crossref] [PubMed]
- Travison TG, Araujo AB, Esche GR, et al. The relationship between body composition and bone mineral content: threshold effects in a racially and ethnically diverse group of men. Osteoporos Int 2008;19:29-38. [Crossref] [PubMed]
- Van Langendonck L, Claessens AL, Lefevre J, et al. Association between bone mineral density (DXA), body structure, and body composition in middle-aged men. Am J Hum Biol 2002;14:735-42. [Crossref] [PubMed]


