
Clinical presentations of Wilson disease
Introduction
Wilson disease (WD) is an autosomal-recessive disorder of copper metabolism caused by mutations in the ATP7B gene (1,2). It presents in childhood, adolescence or adulthood with a wide range of clinical manifestations. The disease prevalence has previously been estimated as 1 in 30,000 (3,4), but some recent analyses have suggested a genetic prevalence of 1 in 7,000 (5,6).
Copper is absorbed from the stomach and duodenum, taken up by the liver, and secreted by the liver into the systemic circulation bound to ceruloplasmin (7). ATP7B transports copper through the trans-Golgi network in hepatocytes before it is incorporated into apoceruloplasmin which is secreted as holoceruloplasmin. ATP7B is also essential for biliary excretion of copper when cytoplasmic levels are high. Dysfunction of ATP7B therefore leads to accumulation of copper in the liver giving rise to cellular damage and disease, and the release of non-ceruloplasmin bound copper into the systemic circulation. Copper also accumulates and is associated with cellular damage and disease in other organs, most notably the brain (8). The extent to which disease in the brain relates to high levels of free circulating copper and/or underlying dysfunction in neurons, which also express ATP7B (9), is not clear.
Originally referring to this condition as progressive lenticular degeneration, Samuel Alexander Kinnier Wilson first described the combination of neurologic disease with cirrhosis in 1912 (10). He recognised that psychiatric manifestations were common but stated that the cirrhosis was rarely symptomatic during life (10). Barnes and Hurst subsequently reported in 1925 that WD can present with symptomatic liver disease in the absence of neurologic features (11). It is now accepted that symptomatic involvement of the liver or brain can occur in isolation or in combination at presentation. Asymptomatic, or presymptomatic, liver and brain disease in siblings can be identified and studied through family screening (12).
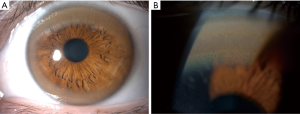
A very important ophthalmologic feature of WD is the Kayser-Fleischer (KF) ring (Figure 1). Copper is deposited in Descemet’s membrane within the cornea and may be visible as a greenish-brown opacity at the periphery of each iris (13). They may not be seen in all patients, but are virtually pathognomonic of WD if present. Clinical features in other organs have also been described, including osseomuscular, renal, endocrine and cardiac manifestations, although these are rarely the presenting features (14-17).
The challenge in recognising WD
Chelating agents, such as D-penicillamine and trientine dihydrochloride, and zinc salts usually improve symptoms and prevent disease progression and death (18-21). The importance of recognising WD at the initial presentation cannot therefore be overemphasised. The consequences of a late or missed diagnosis are of disease progression, which may be fatal. However, diagnosis is often delayed:
The mean delay from symptom onset to diagnosis is around 2 years (20,22). Merle et al. showed that this delay is greater in neurologic than hepatic presentations (44 vs. 14 months). A delay of up to 30 years was also reported (20). In a study from the UK, the mean delay from initial presentation to diagnosis was 13 months in patients in whom the correct diagnosis was not made initially, illustrating that the problem is not simply related to delayed presentation to a clinician (23).
There are several reasons why WD may not be considered at the initial clinical assessment:
- WD is rare and the initial manifestations in the majority of cases have numerous mimics that are much more prevalent. Even clinicians within the principal specialties involved may not have previously diagnosed a case of WD.
- The clinical features of both hepatic and neurologic presentations are highly variable: Walshe and Yealland explained that ‘no two patients are ever the same, even in a sibship’ (23).
- KF rings are absent in up to 45% of hepatic and 10% of neurologic presentations and may only be visible with slit lamp examination (24).
- Neurologic manifestations may be subtle and easily missed by patients presenting to hepatologists, and vice versa.
- Psychiatric symptoms, although common, are also prevalent in the general population and can easily be dismissed as unrelated, or as a reaction, to the presenting symptom (25,26).
The problem of delayed diagnosis is compounded by the lack of a single reliable diagnostic test for WD. Some physicians may be over-reliant on specific tests, for example serum ceruloplasmin levels, which may be normal in 40% of hepatic and 15% of neurologic presentations (Table 1) (24). After considering the possibility of WD, a wide range of tests, some of which take extra effort to complete or are invasive, may be required to make the diagnosis. Apart from serum ceruloplasmin, a serum copper, 24-hour urinary copper output, slit lamp examination (by an experienced ophthalmologist), brain MRI, liver biopsy with copper quantification (histology may suggest but does not rule out WD) and ATP7B genotyping may be required (27). A combination of only a few of these, for example a low ceruloplasmin and the presence of KF rings, may give the secure diagnosis. Ferenci et al. have published a scoring system (often referred to as the Leipzig score) using these features that allows an assessment of whether a diagnosis of WD is highly likely, probable or unlikely (Table 2) (28). Diagnostic algorithms have been derived from this (2).

Full table

Full table
The onus is therefore on physicians to use their clinical skills to think of WD as a possible diagnosis and to determine when and how to embark on testing for WD. This review will describe the range of clinical presentations, concentrating on hepatic and neurologic disease, in order to aid the timely investigation for and diagnosis of this progressive and potentially fatal disease. Direct antiglobulin (Coombs) test negative hemolysis, which can be a vital clue to the diagnosis, will be briefly mentioned, as will psychiatric manifestations, but other organ involvement and the interpretation of investigations will be covered in other contributions. One particular aspect that we emphasise throughout is that combinations of specific clinical features provide red flags which should alert clinicians to consider WD when assessing any patient presenting with hepatic or neurologic disease.
Classification of presentations
WD has, historically, been classified into neurologic, hepatic, mixed or asymptomatic presentations. Dening et al. used a cluster analysis to provide supportive evidence for these four subgroups in a combined cohort of 400 cases in 1989 (29). Psychiatric symptoms were not included as input variables and did not therefore feature in the resulting classification. These are common and under-recognized in WD and some authors therefore refer to ‘neuropsychiatric’ presentations to denote the presence of neurologic or psychiatric features.
A working party at the 8th International Meeting on WD in Leipzig in 2001 revised the phenotypic classification and differentiated cases into neurologic (N), hepatic (H) or other (O) presentations (28). Any patients in whom neurologic and/or psychiatric symptoms are present at the time of diagnosis are classified as a neurologic presentation. They are then subdivided into those with (N1) or without (N2) symptomatic liver disease, or not investigated for liver disease (Nx). This classification requires a detailed neurologic examination to exclude neurologic symptoms at diagnosis and a liver biopsy to confirm the absence of marked liver disease. Hepatic presentations are subdivided into acute (H1) or chronic (H2) depending on the presence of acute jaundice due to hepatitis and/or hemolysis in a previously healthy subject (H1), or any type of chronic liver disease, with or without symptoms (H2). The presence of any biochemical evidence of liver disease indicates a hepatic (H), as opposed to other (O), presentation.
The relative frequency of neurologic and hepatic presentations has been examined in several large cohorts over the last three decades. The proportion of patients that would, under the 2001 classification, be referred to as neurologic, either N1 or N2, ranges from 37% to 80% (14). While there is likely to be some variation in phenotype between individual populations, comparing these cohorts may be problematic for other reasons.
Firstly, selection bias may affect the relative frequency of different presentations in individual cohorts; centres of excellence for neurology are likely to report a higher number of cases with neurologic involvement. Secondly, neurologic and hepatic features may be subtle or identifiable only through specific investigations. The classification of presentations may therefore have been inconsistent in some cohorts, especially before the introduction of rating scales for WD such as the Unified Wilson’s Disease Rating Scale in 2008 and the Global Assessment Scale in 2009 (30,31).
Age and gender distributions
The presentation of WD varies according to age and gender. Merle et al. (20) reported the mean age of symptom onset as 15.5 years for those with hepatic and 20.2 years with neurologic involvement. Litwin et al. (22) reported gender differences in 627 consecutive WD cases. Neurologic presentations were more common at diagnosis in men than women (60% vs. 39%) and hepatic presentations were more common in women than men (58% vs. 41%).
These patterns may help inform our understanding of the pathophysiological basis for phenotypic variation but should not influence decisions to investigate WD. While the majority of cases present between the age of 5 and 35 years old (32), the range is wide for both hepatic and neurologic presentations from. A diagnosis of WD has been reported in a 3-year-old child and in two individuals in their eighth decade of life (33,34).
Hepatic presentations
These range from acute presentations, acute hepatitis to acute (fulminant) liver failure, and chronic presentations from steatosis and chronic hepatitis to compensated and decompensated cirrhosis. Several clinical features are relevant to all hepatic presentations and should immediately raise the suspicion of WD.
Firstly, there may be a family history of liver or neurologic disease among siblings. Presentation can differ between siblings and clinicians should also be aware that pseudo-dominant inheritance can occur and enquire about the proband’s parents, including any history of consanguinity.
Secondly, KF rings may be visible at the bedside. Even if the index of suspicion for WD is low, the specialist should examine to check whether KF rings (Figure 1) are present if the cause of liver disease is unclear or if other clinical features of WD are suspected. Slit lamp examination by an experienced ophthalmologist would be necessary to rule out KF rings.
However, pre-existing or emerging neurologic and/or psychiatric features (discussed in more detail below) can be seen in any hepatic presentation. It is not practical for a hepatologist to perform a comprehensive neurologic and psychiatric assessment in all patients presenting with liver disease however the presence of subtle tremor, drooling or dysarthria in an otherwise alert patient can be easily assessed. Anxiety and mood disorders are prevalent in the general population but asking about change in behaviour or personality and difficulty managing at school or work may help quickly identify other specific cognitive and/or psychiatric problems.
Acute hepatic presentations
WD patients, predominantly children or young adults, may present with an acute hepatitis similar to a viral hepatitis. This may present as, or progress to, a more severe acute liver injury (ALI) associated with a coagulopathy or acute liver failure (ALF) characterised by the development of hepatic encephalopathy (HE) (35). Classically there is also acute kidney injury (renal failure).
However, several definitions for ALF, also referred to as fulminant liver failure, have been described (36). The classification proposed by O’Grady suggests that hyperacute, acute and subacute liver failure be differentiated by the time between the onset of symptoms (usually jaundice) to the onset of the encephalopathy, referring to 1–7 days, 8–28 days and 5–12 weeks, respectively (37). Technically, these definitions require the absence of pre-existing liver disease however an exception is made for de novo cases of WD (in which cirrhosis may be present) given the clinical picture and poor prognosis are similar to other etiologies (35). Otherwise, these presentations would be defined as acute-on-chronic liver failure (35).
In a large cohort of 308 consecutive patients presenting with ALF to tertiary care centers, WD accounted for eight cases (2.6%), of which one, five and two were hyperacute, acute and subacute, respectively (38). Two died before transplantation and the remainder had liver transplantation. The most common causes of ALF included viral and drug-induced hepatitis. In other cohorts, WD accounts for up to 9% of cases and, similar to other etiologies, was more common in females (39).
Dhawan et al. have published a cohort that included 57 children presenting with symptomatic WD (40). According to the definition above, 17 of the 57 patients presented with ALF (with encephalopathy) and 10 presented with ALI (without encephalopathy). Common features across all presentations were jaundice, abdominal pain, ascites and hepatosplenomegaly. Other presenting features were peripheral edema, dark urine, fever, epistaxis, pruritus, gynaecomastia and joint pain. Of the 15 patients that died, two had ALI, highlighting the need for early identification of cases without encephalopathy that require transplantation. The authors address this by revising and validating the Wilson Index for predicting mortality without liver transplantation in this scenario. This uses serum bilirubin, international normalised ratio (INR), aspartate aminotransferase and white cell count (40).
It is important to make a diagnosis of WD in patients with acute hepatic presentations in order to provide optimal management, monitor for specific complications of WD (41,42), and highlight the need for family screening. Chelation therapy has been reported to be effective in cases of ALI (43), although it is important not to miss the opportunity to perform transplantation when clinically indicated. Diagnostic biochemical or genetic tests may not be feasible in the time available and the physician may need to depend on the clinical assessment and initial investigations to make the diagnosis.
A hemolytic anemia with a negative direct antiglobulin (Coombs) test can provide a vital clue to the diagnosis. It is discussed in more detail below but is associated with a poor prognosis and sometimes referred to as a Wilsonian crisis in the context of ALF. Dhawan et al. reported that it occurred in 30% of pediatric cases presenting with ALF that required transplantation and 60% of those who died prior to transplantation (40). An anemia or disproportionately high bilirubin level, caused by the combination of hepatocellular injury and red cell destruction (44,45), may be evident on initial bloods tests and should prompt further investigations with reticulocyte count, direct antiglobulin (Coombs) test and haptoglobin level.
A low alkaline phosphatase (ALP), low ALP to bilirubin ratio or high AST to ALT ratio should also raise suspicion of WD. An ALP (IU/L) to total bilirubin (mg/dL) ratio of less than 4 has been reported as having a sensitivity of 94% and specificity of 96% for WD in this setting, and an AST to ALT ratio of more than 2.2 has been reported as having a sensitivity of 94% and a specificity of 86% (46,47). However, these findings are not seen in all cases (48). Serum urate has also been reported to be low in patients with hepatic and neurologic presentations of WD, although whether this is a specific finding is unclear (15).
KF rings are present in more than half of cases presenting with ALF (40). Slit lamp examination may be challenging in the intensive care setting but WD should be considered in any patient with unexplained ALI or ALF, or the above clues to the diagnosis.
Neurologic symptoms or signs can occur in parallel with ALF due to WD or emerge while patients are being considered for transplantation. However, it is important to differentiate these from HE, which is defined as ‘brain dysfunction caused by liver insufficiency and/or porto-systemic shunting manifesting with a wide spectrum of neurologic or psychiatric abnormalities ranging from subclinical alterations to coma’ (49). The West Haven criteria are often used to grade the severity of HE in a clinical setting and describe the core features of HE (Table 3) but are problematic in the context of a patient with WD given the majority of features in grades I and II can be seen in neurologic presentations of WD without significant liver disease. Hepatologists should however be aware that tremor, drooling and/or dysarthria in an otherwise alert patient with ALF are red flags for WD at any age.

Full table
If the results of biochemical tests for WD are available, it is important to recognise that these can be misleading in the acute setting. The serum copper level, which represents both ceruloplasmin-bound and non-ceruloplasmin-bound copper, is usually low at the time of diagnosis in non-acute presentations of WD but is likely to be high in WD-related ALF as non-ceruloplasmin-bound copper is released from the failing liver. The urinary copper output (if available) should also be very high. Serum ceruloplasmin, which is an acute phase reactant, may be normal or raised and a low serum ceruloplasmin has a sensitivity of 21–56% and specificity of 63–84% for the diagnosis of WD in the context of ALF (46). It should also be noted that the calculation of non-ceruloplasmin-bound copper may be inaccurate if the serum ceruloplasmin is raised.
Chronic hepatitis
In any patient referred with chronic transaminitis a range of diagnoses will be considered in the clinical assessment and initial investigation. In addition to the combination of clinical features referred to earlier, a history of hemolysis, or previous unexplained episodes of jaundice, may be suggestive of WD. An alcohol history will be taken routinely but alcohol excess should not be used to exclude the diagnosis of WD or explain a tremor (except in overt alcohol withdrawal). Screening investigation includes serology for hepatitis B and C, an autoantibody screen looking for features of autoimmune hepatitis, iron studies (ferritin and transferrin saturation), alpha-1-antitrypsin, a liver ultrasound and importantly a serum ceruloplasmin. Transient elastography can give a measure of hepatic fibrosis.
If the serum ceruloplasmin is low, slit lamp examination for KF rings and a urine collection to measure copper excretion, are performed. Depending upon the outcome of these tests the decision will be made whether to proceed with liver biopsy and liver copper measurement and/or ATP7B genotyping. A borderline low serum ceruloplasmin should lead to a full screen for WD as serum ceruloplasmin may be normal in hepatic WD.
The literature has drawn attention to the fact that the clinical picture including liver biopsy may suggest an autoimmune hepatitis (50), presenting at a similar age with jaundice, raised transaminases and gamma globulins, although autoantibodies are negative. Patients are reported who were initially diagnosed with and treated for autoimmune hepatitis and subsequently were diagnosed with WD, and the authors recommended thorough screening for WD in patients with an initial diagnosis of autoimmune hepatitis (51). Measurement of serum ceruloplasmin in these patients is part of the hepatic ‘database’ (but may be normal). Further investigation with other tests is particularly important in those patients not responding as expected to immunosuppressive treatment.
Throughout the spectrum of WD, it must be emphasised that although histology may have features suggestive of WD (rhodanine-positive staining for copper; glycogen vacuolation of hepatocyte nuclei), broadly the features are non-specific, and liver histology appearances are never able to rule out WD. Indeed, Ferenci et al. report minimal changes or normal appearances in 3–10% of their series of patients (52). If liver biopsy is being done then liver copper quantification is essential.
Cirrhosis
Cirrhosis is characterised by a compensated phase during which time the patient is asymptomatic and liver disease may progress undetected. This is followed by a decompensated phase during which increasing portal pressure and worsening liver function precipitate ascites, gastrointestinal bleeding, encephalopathy or jaundice. Decompensated cirrhosis can be further complicated by acute kidney injury, hepato-renal syndrome, hepato-pulmonary syndrome, porto-pulmonary hypertension, cirrhotic cardiomyopathy, relative adrenal insufficiency and bacterial infections, including spontaneous bacterial peritonitis. Liver disease accelerates towards liver failure and death in the decompensated phase of cirrhosis (53).
As in chronic hepatitis the range of causes is wide for this scenario, but the screening for causes should include tests for WD. The asymptomatic patient with compensated cirrhosis may have features of chronic liver disease with spider naevi, splenomegaly and thrombocytopenia due to portal hypertension. In such patients all causes of liver disease need to be considered, with a database screen performed (as under chronic hepatitis).
Again, serum caeruloplasmin analysis should be requested but may be normal, and other tests for WD may be required, particularly with a positive family history, previous episodes of hemolysis or unexplained neurologic or psychiatric symptoms.
Steatosis
Steatosis, or fatty change, is seen in around 15% of liver biopsies taken from patients with WD (52). In children there are reports showing steatosis in 50–80% on liver histology (54). These are features of WD but could be one aspect of the clinical presentation, in, for example, a patient with abnormal liver tests. Here, screening for WD would be part of the initial investigation. Dissecting out the patients with WD from the increasingly frequent clinical scenario of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), due to metabolic disease and insulin resistance, is a challenge. However, ruling out WD before making the diagnosis of NAFLD in a child or adult is considered very important (55) because of the implications for management.
Other liver disease
While WD can mimic other diseases, and vice versa, clinicians should be aware of the possibility of multiple diagnoses. The clinical features of patients with both chronic hepatitis B and C infections and WD have been described (56). A previous report recorded the clinical features of 9 patients with WD as well as concurrent chronic hepatitis C, haemochromatosis, primary biliary cholangitis or primary sclerosing cholangitis. Two patients developed hepatocellular carcinoma. This group of nine patients were older at diagnosis of WD and had more severe liver disease (perhaps because of delayed diagnosis) and a greater mortality (57). Mutation analysis was considered crucial for definitive diagnosis in over 50% of these patients. Apart from the severity of liver disease, such patients raise issues with regard to WD treatment—disease progression despite optimal treatment, or overtreatment because of progression due to unrecognised concomitant disease.
Although hepatocellular carcinoma is reported in patients with WD (58), this is generally considered to be a lower risk than in patients with other causes of cirrhosis, and may be related to concomitant liver disease, as described above.
Hemolysis
In a classic review of over 300 cases of WD by Walshe, 22 patients (7%) had a history of acute haemolytic syndrome. The mean age of onset was 12.6 years (59). Diagnosis of WD was delayed in 18 of 22 patients and resulted in progression to severe liver disease in 14 and neurologic disease in 4 patients. The reason for a correct diagnosis being made was progressive liver damage in 10, development of neurologic signs in 6 and diagnosis of a sibling in 2 patients. The message is therefore that the possibility of WD is not recognised in the majority.
In those seen during the episode, the features were of anemia, a raised reticulocyte count, negative direct antiglobulin (Coombs) test and hyperbilirubinemia, with conjugated versus unconjugated bilirubin around 50–70%, presumably because of concomitant liver disease. Copper studies were characteristic of WD with a high non-ceruloplasmin-bound copper.
Neurologic presentations
The majority of neurologic presentations consist of a movement disorder associated with bulbar symptoms.
The movement disorder is usually characterised by tremor, dystonia or parkinsonism. These ‘core’ movement disorders often occur in combination and may initially be subtle. Bulbar symptoms consist of dysarthria, drooling and/or dysphagia. There are a range of additional neurologic features, including cerebellar dysfunction, chorea, hyperreflexia, seizures and cognitive impairment, which can also co-exist, in addition to psychiatric features (Table 4).

Full table
The presence of bulbar symptoms, a mixed movement disorder, cognitive impairment and associated psychiatric features are therefore all red flags for WD.
Classification of neurologic features
Several different classifications of neurologic presentations have previously been proposed and this reflects the complexity of the features seen in WD.
Denny-Brown initially referred to two subgroups: one with predominant tremor referred to as pseudosclerosis and another with predominant dystonia referred to as progressive lenticular degeneration (60). However, Scheinberg and Sternlieb reported that while the majority of WD cases have tremor or dystonia ‘in reality almost every patient with neurologic signs and symptoms suffers from tremulousness and dystonia, although one or the other usually dominates’ (4). Walshe and Marsden separately described the third category, which they referred to as parkinsonian (23,61,62).
Członkowska et al. recently studied the clinical features of 53 treatment-naïve WD patients with neurologic presentations by applying the Unified Wilson’s Disease Rating Scale (UWDRS) (63). Dysarthria was the most common individual neurologic feature. Participants were further classified into predominant neurologic syndromes by experienced neurologists; 62% exhibited tremor, 15% were dystonic and 11% had parkinsonism. Discrete or unclassified signs were seen in 11%.
We will describe bulbar symptoms, tremor, dystonia and parkinsonism in presentations of WD before summarising additional neurologic features, including cognitive impairment, and psychiatric features. A more comprehensive discussion of each individual clinical feature is available in several recent chaptersJeny (64,65). As before, a positive family history, a suspicion of KF rings (seen in more than 90% of neurologic cases), and any evidence of liver disease should immediately raise the possibility of WD, regardless of the neurologic presentation. Physicians should specifically ask about any previous episodes of jaundice or liver disease, which is reported in 23–30% of neurologic cases (14,23), and be aware that unexplained thrombocytopenia or coagulopathy may indicate undiagnosed liver disease.
Bulbar symptoms
Dysarthria was described in all 12 of SAK Wilson’s original cases where it progressed to more or less complete anarthria in the absence of treatment (10). It is seen in 73–91% of cases with neurologic presentations (63,66,67). It is a cardinal feature of neurologic WD and can be related to combination of dystonia, parkinsonism, cerebellar dysfunction or spasticity (68,69). Thus patients with WD may also have difficulty with initiation, intonation and/or volume of speech in addition to dysarthria, which specifically refers to impaired articulation.
Dysarthria is of particular importance to clinicians because it often presents earlier in the disease course, can be easily identified (by patients, their family and physicians) and can provide a vital clue to the diagnosis (23). Walshe and Yealland retrospectively analysed the symptoms and signs at the initial presentation to a physician in 65 children and 71 adults with neurologic presentations (23); speech disturbance was one of the initial symptoms in 71% and one of the initial signs in 79% of cases. While dysarthria has a broad differential diagnosis, it does not occur in the early stages of most other common causes of dystonia, tremor or parkinsonism and should be a red flag for WD in any patient presenting with a movement disorder or liver disease.
Drooling, or sialorrhea, is also an early feature of WD and is seen at the initial examination in up to 21% of neurologic cases (23). Trocello et al. assessed drooling in 10 consecutive symptomatic patients and identified problems with reduced swallow frequency, longer swallow latency and poor swallowing capacity. Oropharyngeal sensitivity disorders were present in half of cases (70).
Dysphagia is an early symptom in 14% of patients with neurologic presentation and, like dysarthria, can provide an important clue to the diagnosis (23). It is also a major potential cause of mortality if the disease progresses due to the risk of aspiration pneumonia. Scintigraphy studies have confirmed that the dysphagia is associated with slower oral transit and greater oral residue, even in some patients without neurologic symptoms (66,71).
Tremor
Tremor can be divided into postural, rest or kinetic on the basis of activating conditions.
Postural tremor of the upper limbs, assessed by asking the patient to hold both arms outstretched, is the most common movement disorder in WD; ‘hand tremor’ was one of the initial symptoms in 70% of the cohort reported by Walshe and Yealland (23). This can mimic essential tremor, a much more prevalent movement disorder that also occurs in young people. Tremor in WD, like dystonia and parkinsonism, can also be asymmetrical (72). Wing-beating tremor refers to a large amplitude postural tremor in the upper limbs that emerges as the elbows are flexed. This is claimed to be a specific feature of WD but is more an indication that the tremor is associated with dystonia and can have a number of causes.
Rest tremor, which is classically associated with parkinsonism, appears to be less common in WD. Leinweber et al. described a cohort of 107 patients on treatment for WD using the UWDRS and reported that it was seen in 11% of cases (30). Difficulty with the finger-to-nose test, suggestive of a kinetic tremor was seen in 17% and a head tremor was seen in 6% of cases. The combination of a postural, rest and kinetic tremor, often referred to as a Holmes’ or rubral tremor, has also been reported in WD (73).
Tremor can affect handwriting early in the disease course and it may be helpful to ask patients about this and observe them writing a sentence and copying an Archimedes spiral. Handwriting can also be affected by dystonia and parkinsonism (discussed below).
All patients presenting with a postural tremor should be examined for KF rings and have serum ceruloplasmin and copper levels analysed. A subacute history, progression over the following months or any associated psychiatric symptoms should prompt an immediate and comprehensive screen for WD, including slit lamp examination, 24-hour urine collection and MRI of the brain, as should any features of previous or ongoing liver disease.
Dystonia
The term dystonia can be misleading as it can be used to describe a symptom, sign, syndrome or diagnosis. It was recently defined as a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures or both (74). Confusingly, dystonia can also precipitate a jerky rest, postural or kinetic tremor and the term dystonic tremor is often used by neurologists to refer to the combination of dystonia and tremor within the same body area.
While dystonia is the predominant feature in only around 15% of WD cases at presentation it is seen to a lesser degree in the majority of cases; Machado et al. described dystonia in 69% of a series of patients with neurologic presentations (66). Involuntary hyperextension of the digits or wrist or slight tilting or turning of the head as the patient holds their arm outstretched and/or in elbow flexion can be subtle but provide an important clue towards a prompt diagnosis of WD.
Dystonia of the facial and oromandibular muscles provokes a grimacing appearance, often referred to as risus sardonicus, and is reported in 10–72% of WD cases (23,66). This may also be subtle and difficult to identify in the absence of other signs. Difficulty in performing alternating lateral tongue movements (and dysarthria) may provide supportive evidence of oromandibular dystonia.
We would advocate investigating all presentations of dystonia with serum ceruloplasmin and copper levels and an MRI of the brain. An exception is isolated cervical dystonia, which is a relatively common movement disorder in adults that presents with laterocollis and/or torticollis, with or without a jerky head tremor, and is unlikely to be caused by WD (27). In the context of a subacute presentation or any presentation of dystonia with dysarthria we advocate a full screen for WD that includes a slit lamp examination and 24-hour urine collection for copper in the first instance.
Parkinsonism
The frequency of parkinsonism, or pseudoparkinsonian features, as a presentation of WD is variable between cohorts. As discussed above, Członkowska et al. (63) reported that it is the predominant feature in only 11% of newly-diagnosed neurologic presentations. In contrast, Walshe and Yealland (23) and then Taly et al. (14) reported that parkinsonism accounted for 45% and 62% of presentations in their cohorts, respectively.
These discrepancies are probably related to changes in the definition of parkinsonism over the last few decades. The most recent definitions require the presence of limb bradykinesia, a specific type of slowness associated with decrement in the amplitude or speed, or progressive hesitations, as movements are continued (75). It can be assessed with repeated finger and foot tapping. Other features of parkinsonism include rest tremor, rigidity, postural instability, an abnormal gait, hypomimia (reduced facial expression) and micrographia.
While parkinsonism in isolation, or as the predominant feature, appears to be relatively rare in WD it is commonly seen in combination with dystonia or tremor at presentation. Machado et al. have reported that ‘pure parkinsonism’ occurred in less than 2% of neurologic presentations but that parkinsonism in combination with other movement disorders is seen in the majority; bradykinesia was reported in 58% of their cohort (66).
We advocate a full screen for WD in any patient presenting with parkinsonism in childhood, adolescence or early adulthood. Anyone presenting with parkinsonism that is subacute or progressing over months or with concomitant dystonia or prominent dysarthria should also be promptly investigated.
Other movement disorders
Cerebellar dysfunction is characterised by a broad-based gait, impaired tandem walking, dysarthria, dysmetric limb movements (finger-nose or heel shin ataxia), intention tremor (a specific type of kinetic tremor that increases as the finger approaches the target), dysdiadochokinesia and/or a constellation of abnormal eye movements, including nystagmus (76). Similar to parkinsonism, it is frequently reported in large cohort studies of WD, but usually in the context of patients with a postural tremor. Machado et al. reported cerebellar dysfunction in 28% of cases (66). Interestingly, WD was not seen in a cohort of 1,500 cases of progressive cerebellar diseases seen at a large neuroscience centre over 20 years (77) suggesting that isolated cerebellar dysfunction (without tremor, dystonia or parkinsonism) is unlikely to be caused by WD.
Chorea, an ongoing random-appearing sequence of one or more discrete involuntary movements, can be difficult to differentiate from dystonia in some situations. The movements in chorea are less predictable and appear to flow between body regions. Dystonia, often described as athetosis in pediatric literature, is characterised by more sustained and sometimes continuous muscle spasm, often accompanied by posturing (78). Chorea has been reported in 9–16% of patients with WD (14,66), but again, in the context of other concomitant movement disorders.
Spasticity is not usually associated with WD in the absence of advanced disease but hyperreflexia is reported in up to 8% of patients with neurologic presentations at the time of diagnosis (66).
Gait disturbance in WD can be related to underlying dystonia, parkinsonism, cerebellar dysfunction, chorea or spasticity or a combination of these and is one of the initial symptoms in 35% of neurologic presentations (23). There is no specific gait pattern that is typical of WD and some patients present with bizarre or unusual gaits that can be misdiagnosed as a functional (psychogenic) neurologic disorder.
Rarer neurologic presentations
Seizures are reported as the initial symptom of WD in 4–7% of cases (14,29,66). There are a few examples of refractory epilepsy and status epilepticus in the literature but it is rare in WD (29,79). Other unusual presentations include muscle cramps, undulating tongue movements, oculogyric crises, optic neuropathy and an unusual cough due to involuntary respiratory muscle contraction (80-84).
Cognition
This can be affected early in the disease course but any impairment is usually subtle. It may reveal itself through poor performance at school or work, and was one of the initial symptoms in 26% of children in one cohort (23). Neuropsychological assessments in treated patients suggest that the most significant difficulties relate to frontal lobe and subcortical cognitive functions (85), and that language and memory are relatively preserved (86). Patients may have difficulties with attention, impulsivity, emotional lability or executive function, for example planning or decision-making (87). Profound cognitive impairment resulting in dementia appears to be very rare at presentation in the absence of severe neurologic involvement.
Psychiatric features
These are common, affecting 30–40% of WD patients at diagnosis (25), and up to 20% of patients were reported to have seen a psychiatrist prior to diagnosis in one cohort (26). In a systematic review of psychiatric symptoms in WD, Mura et al. reported that decrease in scholastic performance, behavioural problems, affective disorders and psychosis, typically with persecutory delusions, were the most common psychiatric features at onset (26). Similarities with bipolar affective disorder in the form of manic/hypomanic episodes, irritability and disinhibited or aggressive behaviour have also been described (66,88). Psychiatric features of WD are covered in more detail in another contribution but may be observed or easily assessed during a hepatic or neurologic consultation and are therefore only briefly mentioned here.
Asymptomatic patients
This refers to patients without symptoms who are most often, if not universally, diagnosed with WD through family screening but who also require treatment. These patients often have asymptomatic hepatomegaly, abnormal liver function or architecture (fibrosis, cirrhosis) or subtle neurologic or psychiatric signs and thus need to undergo full clinical evaluation after diagnostic testing. Some may have undiagnosed symptoms and the term asymptomatic, or pre-symptomatic, may therefore be inaccurate or misleading.
Walshe et al. reported the clinical, biochemical and imaging findings in 30 patients diagnosed through family screening (12). Limited clinical data were available on six patients that had already started treatment. Of the remaining 24 patients, eight had hepatomegaly or splenomegaly, of whom two had abnormal neurologic signs. Transaminitis was seen in 13 patients and imaging abnormalities were reported in all five patients that had neuroimaging. It was unclear how many had a seizure. Chabik et al. reported on 95 siblings diagnosed from 73 index cases through family screening, 55% of whom were symptomatic. Of 35 siblings of hepatic index cases, 51% had hepatic and 17% had neurologic symptoms and signs (89). Of 60 siblings of neurologic index cases, 23% had hepatic and 23% had neurologic features. While the authors conclude that there is high intra-familial concordance, these findings also support previous reports that presentations can vary between siblings (90).
Conclusions
This review discusses the broad range of hepatic and neurologic presentations of WD in order to aid timely investigation and diagnosis. The challenge of recognising WD early in the disease course, particularly in the context of much more prevalent mimics, is exacerbated by the incomplete sensitivity of the various diagnostic tests. We have therefore tried to list the other potentially associated clinical features for each presentation, and summarise these clinical hints in Table 5.
Full table
A recurring theme is that a combination of hepatic, neurologic, psychiatric and hemolytic manifestations can be identified at presentation but may be subtle and easily missed. Clinicians must therefore be aware of the red flags that lie outside their own discipline when assessing any patient with liver disease, a movement disorder or psychiatric illness and have a low threshold for sending a thorough screen for WD. If doubt exists over the significance of any subtle features then early input from the relevant specialist can be invaluable. We strongly advocate a multi-disciplinary approach to the investigation and workup of suspect WD, including those patients diagnosed through family screening who often show neurologic and/or hepatic features.
Acknowledgements
We are very grateful to the patient who provided the photographs in the picture.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ala A, Walker AP, Ashkan K, et al. Wilson's disease. Lancet 2007;369:397-408. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J Hepatol 2012;56:671-85. [Crossref] [PubMed]
- O'Brien M, Reilly M, Sweeney B, et al. Epidemiology of Wilson's disease in Ireland. Mov Disord 2014;29:1567-8. [Crossref] [PubMed]
- Scheinberg IH, Sternlieb I. Wilson's disease. Major problems in Internal Medicine. XXIII. Philadelphia: WB Saunders, 1984.
- Coffey AJ, Durkie M, Hague S, et al. A genetic study of Wilson's disease in the United Kingdom. Brain 2013;136:1476-87. [Crossref] [PubMed]
- Gao J, Brackley S, Mann JP. The global prevalence of Wilson disease from next-generation sequencing data. Genet Med 2018. [Crossref] [PubMed]
- Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci 2007;30:317-37. [Crossref] [PubMed]
- Faa G, Lisci M, Caria MP, et al. Brain copper, iron, magnesium, zinc, calcium, sulfur and phosphorus storage in Wilson's disease. J Trace Elem Med Biol 2001;15:155-60. [Crossref] [PubMed]
- Davies KM, Hare DJ, Cottam V, et al. Localization of copper and copper transporters in the human brain. Metallomics 2013;5:43-51. [Crossref] [PubMed]
- Wilson SAK. Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver. Brain 1912;34:212. [Crossref]
- Barnes S, Hurst EW. Hepato-lenticular degeneration. Brain 1925;48:279-333. [Crossref]
- Walshe JM. Diagnosis and treatment of presymptomatic Wilson's disease. Lancet 1988;2:435-7. [Crossref] [PubMed]
- Fenu M, Liggi M, Demelia E, et al. Kayser-Fleischer ring in Wilson's disease: a cohort study. Eur J Intern Med 2012;23:e150-6. [Crossref] [PubMed]
- Taly AB, Meenakshi-Sundaram S, Sinha S, et al. Wilson disease: description of 282 patients evaluated over 3 decades. Medicine 2007;86:112-21. [Crossref] [PubMed]
- Wang H, Zhou Z, Hu J, et al. Renal impairment in different phenotypes of Wilson disease. Neurol Sci 2015;36:2111-5. [Crossref] [PubMed]
- Kapoor N, Shetty S, Thomas N, et al. Wilson's disease: An endocrine revelation. Indian J Endocrinol Metab 2014;18:855-7. [Crossref] [PubMed]
- Quick S, Reuner U, Weidauer M, et al. Cardiac and autonomic function in patients with Wilson's disease. Orphanet J Rare Dis 2019;14:22. [Crossref] [PubMed]
- Walshe JM. The conquest of Wilson's disease. Brain 2009;132:2289-95. [Crossref] [PubMed]
- Bruha R, Marecek Z, Pospisilova L, et al. Long-term follow-up of Wilson disease: natural history, treatment, mutations analysis and phenotypic correlation. Liver Int 2011;31:83-91. [Crossref] [PubMed]
- Merle U, Schaefer M, Ferenci P, et al. Clinical presentation, diagnosis and long-term outcome of Wilson's disease: a cohort study. Gut 2007;56:115-20. [Crossref] [PubMed]
- Beinhardt S, Leiss W, Stattermayer AF, et al. Long-term outcomes of patients with Wilson disease in a large Austrian cohort. Clin Gastroenterol Hepatol 2014;12:683-9. [Crossref] [PubMed]
- Litwin T, Gromadzka G, Czlonkowska A. Gender differences in Wilson's disease. J Neurol Sci 2012;312:31-5. [Crossref] [PubMed]
- Walshe JM, Yealland M. Wilson's disease: the problem of delayed diagnosis. J Neurol Neurosurg Psychiatry 1992;55:692-6. [Crossref] [PubMed]
- Steindl P, Ferenci P, Dienes HP, et al. Wilson's disease in patients presenting with liver disease: a diagnostic challenge. Gastroenterology 1997;113:212-8. [Crossref] [PubMed]
- Zimbrean PC, Schilsky ML. Psychiatric aspects of Wilson disease: a review. Gen Hosp Psychiatry 2014;36:53-62. [Crossref] [PubMed]
- Mura G, Zimbrean PC, Demelia L, et al. Psychiatric comorbidity in Wilson's disease. Int Rev Psychiatry 2017;29:445-62. [Crossref] [PubMed]
- Bandmann O, Weiss KH, Kaler SG. Wilson's disease and other neurological copper disorders. Lancet Neurol 2015;14:103-13. [Crossref] [PubMed]
- Ferenci P, Caca K, Loudianos G, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int 2003;23:139-42. [Crossref] [PubMed]
- Dening TR, Berrios GE. Wilson's disease: clinical groups in 400 cases. Acta Neurol Scand 1989;80:527-34. [Crossref] [PubMed]
- Leinweber B, Moller JC, Scherag A, et al. Evaluation of the Unified Wilson's Disease Rating Scale (UWDRS) in German patients with treated Wilson's disease. Mov Disord 2008;23:54-62. [Crossref] [PubMed]
- Aggarwal A, Aggarwal N, Nagral A, et al. A novel Global Assessment Scale for Wilson's Disease (GAS for WD). Mov Disord 2009;24:509-18. [Crossref] [PubMed]
- Boga S, Ala A, Schilsky ML. Hepatic features of Wilson disease. Handb Clin Neurol 2017;142:91-9. [Crossref] [PubMed]
- Wilson DC, Phillips MJ, Cox DW, et al. Severe hepatic Wilson's disease in preschool-aged children. J Pediatr 2000;137:719-22. [Crossref] [PubMed]
- Ala A, Borjigin J, Rochwarger A, et al. Wilson disease in septuagenarian siblings: Raising the bar for diagnosis. Hepatology 2005;41:668-70. [Crossref] [PubMed]
- Wendon J, Cordoba J, Dhawan A, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol 2017;66:1047-81. [Crossref] [PubMed]
- Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013;369:2525-34. [Crossref] [PubMed]
- O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet 1993;342:273-5. [Crossref] [PubMed]
- Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002;137:947-54. [Crossref] [PubMed]
- Gow PJ, Jones RM, Dobson JL, et al. Etiology and outcome of fulminant hepatic failure managed at an Australian liver transplant unit. J Gastroenterol Hepatol 2004;19:154-9. [Crossref] [PubMed]
- Dhawan A, Taylor RM, Cheeseman P, et al. Wilson's disease in children: 37-year experience and revised King's score for liver transplantation. Liver Transpl 2005;11:441-8. [Crossref] [PubMed]
- Medici V, Mirante VG, Fassati LR, et al. Liver transplantation for Wilson's disease: The burden of neurological and psychiatric disorders. Liver Transpl 2005;11:1056-63. [Crossref] [PubMed]
- Guillaud O, Dumortier J, Sobesky R, et al. Long term results of liver transplantation for Wilson's disease: experience in France. J Hepatol 2014;60:579-89. [Crossref] [PubMed]
- Durand F, Bernuau J, Giostra E, et al. Wilson's disease with severe hepatic insufficiency: beneficial effects of early administration of D-penicillamine. Gut 2001;48:849-52. [Crossref] [PubMed]
- Strand S, Hofmann WJ, Grambihler A, et al. Hepatic failure and liver cell damage in acute Wilson's disease involve CD95 (APO-1/Fas) mediated apoptosis. Nat Med 1998;4:588-93. [Crossref] [PubMed]
- Lang PA, Schenck M, Nicolay JP, et al. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med 2007;13:164-70. [Crossref] [PubMed]
- Korman JD, Volenberg I, Balko J, et al. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology 2008;48:1167-74. [Crossref] [PubMed]
- Shaver WA, Bhatt H, Combes B. Low serum alkaline phosphatase activity in Wilson's disease. Hepatology 1986;6:859-63. [Crossref] [PubMed]
- Eisenbach C, Sieg O, Stremmel W, et al. Diagnostic criteria for acute liver failure due to Wilson disease. World J Gastroenterol 2007;13:1711-4. [Crossref] [PubMed]
- Hadjihambi A, Arias N, Sheikh M, et al. Hepatic encephalopathy: a critical current review. Hepatol Int 2018;12:135-47. [Crossref] [PubMed]
- Scott J, Gollan JL, Samourian S, et al. Wilson's disease, presenting as chronic active hepatitis. Gastroenterology 1978;74:645-51. [Crossref] [PubMed]
- Milkiewicz P, Saksena S, Hubscher SG, et al. Wilson's disease with superimposed autoimmune features: report of two cases and review. J Gastroenterol Hepatol 2000;15:570-4. [Crossref] [PubMed]
- Ferenci P, Stremmel W, Czlonkowska A, et al. Age,sex, but not ATP7B genotype effectively influences the clinical phenotype of Wilson disease. Hepatology 2019;69:1464-76. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-60. [Crossref] [PubMed]
- Marcellini M, Di Ciommo V, Callea F, et al. Treatment of Wilson's disease with zinc from the time of diagnosis in pediatric patients: a single-hospital, 10-year follow-up study. J Lab Clin Med 2005;145:139-43. [Crossref] [PubMed]
- Kerkar N, Roberts EA. Wilson Disease in Infancy through Adolescence. In: Kerkar N, Roberts EA. editors. Clinical and Translational Perspectives on Wilson Disease. Academic Press, 2019:179-93.
- Cheung KS, Seto WK, Fung J, et al. Epidemiology and natural history of Wilson's disease in the Chinese: A territory-based study in Hong Kong between 2000 and 2016. World J Gastroenterol 2017;23:7716-26. [Crossref] [PubMed]
- Wong RJ, Gish R, Schilsky M, et al. A clinical assessment of Wilson disease in patients with concurrent liver disease. J Clin Gastroenterol 2011;45:267-73. [Crossref] [PubMed]
- Walshe JM, Waldenström E, Sams V, et al. Abdominal malignancies in patients with Wilson's disease. QJM 2003;96:657-62. [Crossref] [PubMed]
- Walshe JM. The acute haemolytic syndrome in Wilson's disease--a review of 22 patients. QJM 2013;106:1003-8. [Crossref] [PubMed]
- Denny-Brown D. Hepatolenticular degeneration (Wilson's disease). Two different components. N Engl J Med 1964;270:1149-56. [Crossref] [PubMed]
- Walshe JM. Wilson's disease. In: Vinken PJ, Bruyn GW, Klawans HL. editors. Handbook of Clinical Neurology. Vol. 27. Amsterdam: Elsevier, 1976:379-414.
- Marsden CD. Wilson's disease. Q J Med 1987;65:959-66. [PubMed]
- Członkowska A, Litwin T, Dziezyc K, et al. Characteristics of a newly diagnosed Polish cohort of patients with neurological manifestations of Wilson disease evaluated with the Unified Wilson's Disease Rating Scale. BMC Neurol 2018;18:34. [Crossref] [PubMed]
- Członkowska A, Litwin T, Chabik G. Wilson disease: neurologic features. Handb Clin Neurol 2017;142:101-19. [Crossref] [PubMed]
- Aggarwal A, Bhatt M. Neurological Wilson’s disease. In: Kerkar N, Roberts EA. editors. Clinical and Translational Perspectives on Wilson Disease. Academic Press, 2019:195-214.
- Machado A, Chien HF, Deguti MM, et al. Neurological manifestations in Wilson's disease: Report of 119 cases. Mov Disord 2006;21:2192-6. [Crossref] [PubMed]
- Moores A, Fox S, Lang A, et al. Wilson disease: Canadian perspectives on presentation and outcomes from an adult ambulatory setting. Can J Gastroenterol 2012;26:333-9. [Crossref] [PubMed]
- Hefter H, Arendt G, Stremmel W, et al. Motor impairment in Wilson's disease, II: Slowness of speech. Acta Neurol Scand 1993;87:148-60. [Crossref] [PubMed]
- Berry WR, Darley FL, Aronson AE. Dysarthria in Wilson's disease. J Speech Hear Res 1974;17:169-83. [Crossref] [PubMed]
- Trocello JM, Osmani K, Pernon M, et al. Hypersialorrhea in Wilson's Disease. Dysphagia 2015;30:489-95. [Crossref] [PubMed]
- da Silva-Júnior FP, Carrasco AE, da Silva Mendes AM, et al. Swallowing dysfunction in Wilson's disease: a scintigraphic study. Neurogastroenterol Motil 2008;20:285-90. [Crossref] [PubMed]
- Zhou XX, Li XH, Chen DB, et al. The asymmetry of neural symptoms in Wilson's disease patients detecting by diffusion tensor imaging, resting-state functional MRI, and susceptibility-weighted imaging. Brain Behav 2018;8:e00930. [Crossref] [PubMed]
- Tison F, Rouanet F, Neau-Cransac M, et al. Outcome of liver transplantation in Wilson's disease: A demonstrative case. Parkinsonism Relat Disord 1996;2:131-5. [Crossref] [PubMed]
- Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013;28:863-73. [Crossref] [PubMed]
- Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591-601. [Crossref] [PubMed]
- Bodranghien F, Bastian A, Casali C, et al. Consensus Paper: Revisiting the Symptoms and Signs of Cerebellar Syndrome. Cerebellum 2016;15:369-91. [Crossref] [PubMed]
- Hadjivassiliou M, Martindale J, Shanmugarajah P, et al. Causes of progressive cerebellar ataxia: prospective evaluation of 1500 patients. J Neurol Neurosurg Psychiatry 2017;88:301-9. [Crossref] [PubMed]
- Sanger TD, Chen D, Fehlings DL, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord 2010;25:1538-49. [Crossref] [PubMed]
- Türk-Börü U, Kocer A, Alp R, et al. Status epilepticus in a case with wilson's disease during D-pencillamine treatment. Swiss Med Wkly 2003;133:446-7. [PubMed]
- Nagappa M, Sinha S, Saini J, et al. Undulating tongue in Wilson's disease. Ann Indian Acad Neurol 2014;17:225-6. [Crossref] [PubMed]
- Lee MS, Kim YD, Lyoo CH. Oculogyric crisis as an initial manifestation of Wilson's disease. Neurology 1999;52:1714-5. [Crossref] [PubMed]
- Chou LT, Horkey D, Slabaugh M. Acute-Onset Optic Neuropathy in Wilson's Disease. Case Rep Ophthalmol 2019;9:520-5. [Crossref] [PubMed]
- Crone NE, Jinnah HA, Reich SG. Wilson's disease presenting with an unusual cough. Mov Disord 2005;20:891-3. [Crossref] [PubMed]
- Rosen JM, Kuntz N, Melin-Aldana H, et al. Spasmodic muscle cramps and weakness as presenting symptoms in Wilson disease. Pediatrics 2013;132:e1039-42. [Crossref] [PubMed]
- Seniów J, Bak T, Gajda J, et al. Cognitive functioning in neurologically symptomatic and asymptomatic forms of Wilson's disease. Mov Disord 2002;17:1077-83. [Crossref] [PubMed]
- Wenisch E, De Tassigny A, Trocello JM, et al. Cognitive profile in Wilson's disease: a case series of 31 patients. Rev Neurol 2013;169:944-9. [Crossref] [PubMed]
- Lorincz MT. Neurologic Wilson's disease. Ann N Y Acad Sci 2010;1184:173-87. [Crossref] [PubMed]
- Svetel M, Potrebic A, Pekmezovic T, et al. Neuropsychiatric aspects of treated Wilson's disease. Parkinsonism Relat Disord 2009;15:772-5. [Crossref] [PubMed]
- Chabik G, Litwin T, Czlonkowska A. Concordance rates of Wilson's disease phenotype among siblings. J Inherit Metab Dis 2014;37:131-5. [Crossref] [PubMed]
- Takeshita Y, Shimizu N, Yamaguchi Y, et al. Two families with Wilson disease in which siblings showed different phenotypes. J Hum Genet 2002;47:543-7. [Crossref] [PubMed]


