
Long-term impact of prognostic nutritional index in cervical esophageal squamous cell carcinoma patients undergoing definitive radiotherapy
Introduction
Cervical esophageal cancer (CEC) accounts for only 2–10.6% of esophageal cancers, and 95% of CEC cases are classified as squamous cell carcinoma (1). Due to the low incidence and complicated anatomical location of CEC, a consensus has not been reached regarding standard optimal treatment strategies. Conventional surgical resection results in loss of normal digestive function and seriously affects patients’ quality of life (QOL) (2). With major advancements in radiotherapy (RT), intensity-modulated radiation therapy (IMRT) has become commonly used in the treatment of CEC. Moreover, definitive chemoradiotherapy (CRT) has been shown to better protect organ function, leading to a better prognosis for CEC patients, compared with surgical resection and conventional RT in recent studies (3). Despite such significant improvements in the treatment methods for cervical esophageal squamous cell carcinoma (CESCC), the prognosis remains unsatisfactory. The 5-year survival rate for CESCC patients in China was reported to be as low as 30% (1). Therefore, feasible and effective indicators of the response to CESCC treatment with CRT are needed for further individualized treatment selection in order to improve patients’ prognosis.
Recent research demonstrated that the pretreatment nutritional, inflammatory and immunological statuses play crucial roles in the prognosis of solid malignant tumors (4,5). Inflammation-based prognostic factors, including the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR), systemic immunoinflammatory index (SII), and lung cancer inflammation index (ALI) have demonstrated significant prognostic value in many solid malignant tumors (6-10). A nutrition-based prognostic factor known as the prognostic nutritional index (PNI) has also been reported to have predictive value for the prognosis of patients with nasopharyngeal carcinoma (11), hypopharyngeal carcinoma (12), lung cancer (13), breast cancer (14), metastatic urothelial carcinoma (15), and, especially, gastrointestinal cancer (16-19). To our knowledge, however, there have been no studies of the prognostic value of the pretreatment PNI value in patients with CESCC treated with CRT. Therefore, we aimed to determine whether the pretreatment PNI value shows any significant association with the long-term prognosis of CESCC patients treated with definitive CRT.
Methods
Patients
The study was approved by the ethics committee of the Fujian Provincial Cancer Hospital and conducted in accordance with the principles of the Declaration of Helsinki and its amendments. The data for a total of 106 consecutive CESCC patients who were treated with definitive CRT between January 2000 and December 2015 and who met the following criteria were retrospectively reviewed in this study: (I) histologically confirmed CESCC; (II) Karnofsky score ≥70 points; (III) treatment with a RT dose of 50–70 Gy (in 25–35 fractions over a range of 5–7 weeks) with 0–6 courses of platinum-based chemotherapy; (IV) data available from blood biochemical examination 7 days prior to definitive CRT; (V) no history of malignant disease; and (VI) restaged according to TNM staging system issued by the American Joint Committee on Cancer (AJCC; 6th edition, 2002).
Radiotherapy
In this study, 49 CESCC patients received IMRT and 16 patients received three-dimensional conformal radiotherapy (3D-CRT). The cervical and thoracic parts were fixed using one thermoplastic sheet. Imaging data from a prior computed tomography (CT) simulation scan were transmitted to the RT treatment planning system (Pinnacle, version 9.2, Philips Radiation Oncology System, USA), for delineation of the tumor area and the organs at risk. The gross tumor volume (GTV), clinical tumor volume (CTV), and planned tumor volume (PTV) were outlined according to the criteria issued by the National Comprehensive Cancer Network (NCCN). Additional parameters included a prescribed dose of 50–66 Gy, a median dose of 60 Gy, a Bi-lung V20 ≤20%, an average bi-lung dose of ≤20 Gy, a bi-lung V5 of <50%, a heart V30 of ≤30%, and a maximum dose to the spinal cord of <45 Gy.
The other 41 CESCC patients received two-dimensional conventional radiotherapy (2D-CRT). This RT was delivered using anterior and posterior opposing techniques with the lymph node area extending from the subcarinal region to the upper cervical region (anterior-posterior opposed T-shape field) with a prescribed dose of 30 Gy and then off-cord oblique fields at a prescribed dose up to 50–70 Gy.
Chemotherapy
The following chemotherapy regimens were used in some patients: (I) docetaxel 135 mg/m2 D1 + cisplatin 75 mg/m2 D2 or carboplatin AUC 2 D2, or (II) 5-fluorouracil (5-FU) 700–1,000 mg/m2 D1–2 + cisplatin 75 mg/m2 D2.
Definition of inflammation-based indicators
The LMR was defined as the absolute lymphocyte count divided by the absolute monocyte count. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. The PLR was calculated as the absolute platelet count divided by the absolute lymphocyte count. The PNI was calculated by the serum albumin level (g/L) + 5 × absolute lymphocyte count. The SII was defined as the platelet count multiplied by the NLR. Based on previous studies (10), we defined a novel inflammation indicator, the cervical esophageal carcinoma inflammation index (CEI), which was calculated by the body mass index (BMI) multiplied by the serum albumin concentration (g/L)/NLR. The best cutoff values for the LMR, PNI, NLR, PLR, CEI and SII were calculated individually using the Cutoff Finder application.
Follow-up
Follow-up examinations were performed at regular 3-month intervals in the first year, 6-month intervals over the next 2 years, and yearly thereafter. The short-term treatment response was assessed according to the response evaluation in solid tumors (RECIST) criteria at the end of RT. Tumor responses were classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The routine examination included physical examination, blood tests, biological investigations, measurements of tumor markers, thoracic CT scanning, and barium esophagram. We studied the data for all cases and contacted patients by telephone and mail. June 2018 was the last censoring date for the evaluation of survival time. Survival time was measured from the first date of pathologic diagnosis to the date of death or the last follow-up. The median follow-up time was 19.5 months (range, 2–190 months).
Statistical analysis
The main study end point was overall survival (OS). All recorded data were analyzed using SPSS (version 23.0, IBM Corporation, Armonk, NY, USA) and Cutoff Finder application. The Cutoff Finder application was used to calculate the optimal cutoff values for LMR, PNI, NLR, PLR, CEI, and SII. Pearson chi-square test was used to assess the correlation between different categorical variables, and Spearman correlation analysis was used to evaluate the correlation between PNI and other clinicopathological factors. The OS rate was calculated using the Kaplan-Meier method, and a log-rank test was used to assess survival differences between groups. Cox proportional hazards regression analysis was performed to identify independent variables. P values <0.05 indicated statistical significance.
Results
Patients’ characteristics
A total of 106 patients who met the enrollment criteria were included in this study, and their characteristics are presented in Table 1. The median follow-up time was 19 months (range, 2–190 months). Among the 106 patients, 79 (74.53%) were male and 27 (25.47%) were female. The median age was 58 years (range, 41–79 years). There are 40 cases without lymph node metastasis (37.74%) and 66 cases with lymph node metastasis (62.26%). With regard to RT technique, 41 cases (38.68%) patients received 2D-CRT, 16 (15.09%) received 3D-CRT, and 49 (46.23%) received IMRT. Synchronous platinum chemotherapy was administered in addition to RT in 69 cases (65.09%), and 37 cases (34.91%) did not receive chemotherapy (Table 1).
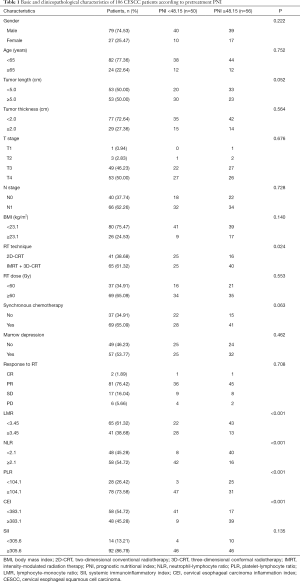
Full table
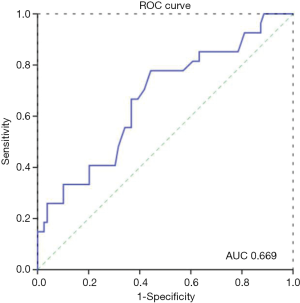
The optimal cut-off value for PNI calculated using Cutoff Finder was 48.15, with an area under the receiver operating characteristic curve (AUC) of 0.669, sensitivity of 77.8%, and specificity of 55.7%. The OS rate was significantly lower in the PNI <48.15 group than in the PNI ≥48.15 group (P=0.004; Figure 1). The optimal cutoff values for LMR, NLR, PLR, CEI, and SII were calculated to be 3.45, 2.1, 104.1, 383.1, and 305.6, respectively.
Relationships between PNI and clinicopathological features
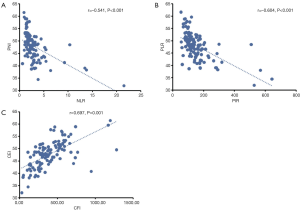
The associations of the PNI and clinicopathological characteristics are presented in Table 2. A low PNI was significantly associated with increased tumor length (P=0.031), increased LMR (P<0.001), increased NLR (P<0.001), increased PLR (P<0.001), and decreased CEI (P<0.001). In additional, there was a negative correlation between PNI and NLR (r=−0.541, P<0.001; Figure 2A) and PLR (r=−0.604, P<0.001; Figure 2B). The PNI was positively correlated with the CEI (r=0.697, P<0.001; Figure 2C).
Full table
Correlation of PNI with survival and prognosis assessment
Overall, the 1-, 2-, 3-, and 5-year OS rates were 75.5%, 46.2%, 39.4%, and 28.3%, respectively. No patients were lost to follow-up, and among all patients who died, the cause of death was cancer related. For the patients with a PNI ≥48.15 (n=56), the 1-, 2-, 3-, and 5-year OS rates were 85.7%, 58.9%, 53.5%, and 41.5%, respectively. The corresponding rates in patients with a PNI <48.15 (n=53) were 64.0%, 32.0%, 25.8%, and 13.5%, respectively. Kaplan-Meier analysis showed that overall the high PNI group had superior OS compared with the low PNI group (P<0.001, Figure 1).
Univariate and multivariate survival analysis
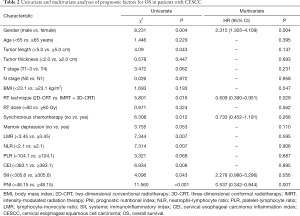
Univariate analysis revealed that gender (P=0.004), tumor length (P=0.043), RT technique (P=0.016), synchronous platinum-based chemotherapy (P=0.012), LMR (P=0.007), NLR (P=0.007), CEI (P=0.008), SII (P=0.043), and PNI (P<0.001) were prognostic factors in CESCC patients (Figure 3). Multivariate analysis demonstrated that gender [hazard ratio (HR) 2.315, 95% confidence interval (CI): 1.305–4.109, P=0.004), RT technique (HR 0.609, 95% CI: 0.390–0.951, P=0.029), and PNI (HR 0.537, 95% CI: 0.342–0.844, P=0.007) were independent prognostic factors in the study patients.
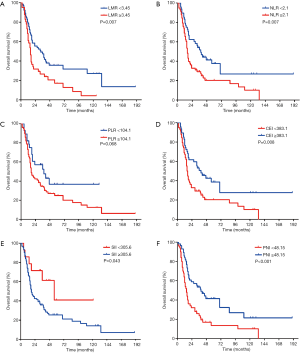
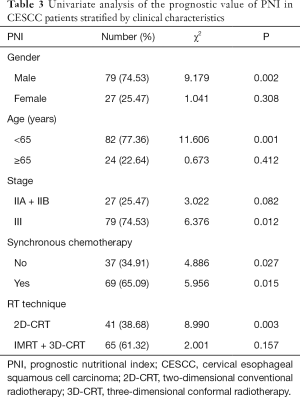
The predictive value of PNI for OS was further assessed after stratification by gender, age, clinical stage, synchronous chemotherapy, and RT technique. PNI was associated with OS only in patients who were male, who were age <65 years, who had stage III disease, who received synchronous chemotherapy, and who were treated with 2D-CRT (Table 3). Stage III patients with a PNI <48.15 had significantly worse OS than those with a greater PNI (HR 6.376; P=0.012). Although stage II patients with a PNI <48.15 also showed worse survival rates than those with a greater PNI, the differences were not statistically significant (HR 3.022; P=0.082).
Full table
Discussion
Recently, many published studies have described a role of pretreatment PNI in the prognosis of solid malignant tumors. However, to our knowledge, no studies have investigated the prognostic value of pretreatment PNI in CESCC. In the present study, we demonstrated the significant predictive value of pretreatment PNI in CESCC patients treated with definitive CRT. Our results showed that a low PNI (<48.15 vs. ≥48.15) was significantly associated with increased tumor length, conventional RT, increased LMR, increased NLR, increased PLR, and decreased CEI. Moreover, a low pretreatment PNI was significantly associated with reduced OS in CESCC patients treated with definitive CRT, and pretreatment PNI was shown to be an independent prognostic factor on multivariate analysis.
In 1980, a study firstly revealed that the nutrition-based prognostic factor, PNI, which is based on the serum album level and lymphocyte count, is strongly associated with OS in gastrointestinal patients treated with radical resection (20). Thereafter, accumulating in vitro and in vivo evidence verified this finding and revealed that the underlying biological mechanisms related to the function of the pretreatment serum albumin and lymphocyte levels included: (I) the pretreatment serum albumin is a crucial nutritional marker, and therefore, a lack of serum albumin represents a poor nutritional status with adverse outcomes (21-24); (II) hypoalbuminemia reflects an increased degree of inflammation, which could negatively impact patients’ survival (25,26); (III) serum albumin plays a crucial role in transporting materials such as cholesterol, fatty acids, metal ions, etc., and therefore, hypoalbuminemia could potentially result in the poor outcomes; (IV) serum albumin scavenges free oxygen radicals, and therefore, a lack of serum albumin leads to negative outcomes (27); and (V) lymphocytes play an important role in the anti-tumor reaction to suppress tumor cell proliferation, migration, and angiogenesis, and thus, a low lymphocyte count can lead to poor outcomes (28-30).
More and more studies have focused on analyzing the relationship between PNI and outcomes in solid tumor patients. Jin et al. (31) studied 1,156 small cell lung cancer patients treated with platinum-based chemotherapy and revealed that the patients with a PNI >53.85 had a better 5-year OS than the patients with a PNI ≤53.85 (24.9% vs. 18.6%). Moreover, PNI was a positive independent prognostic factor for their patients’ OS. Hirahara et al. (32) retrospectively studied 368 gastric cancer patients who were treated with laparoscopic gastrectomy and found that the patients with a low PNI (≤43) had a poorer OS than those with a high PNI. In addition, the PNI was an independent predictor of OS and cancer-specific survival in their study. Okada et al. (13) studied 248 non-small cell lung cancer patients who underwent curative resection and found that a low PNI (cut-off <48) was significantly associated with poor outcomes. In addition, their results showed that PNI was an independent prognostic factor for OS and recurrence-free survival.
In addition to the solid tumors mentioned above, other studies explored the relationship between PNI and ESCC treated with radical esophagectomy. However, no published studies have focused on the relationship between the PNI and outcomes in CESCC patients treated with definitive CRT. Hirahara et al. (33) retrospectively studied 169 ESCC patients who received curative esophagectomy and showed that a PNI >49.2 was associated with a significantly worse OS and that the PNI was an independent prognostic factor for long-term OS. Similar to these findings, Han et al. (34) demonstrated that a PNI >50.75 positively impacted ECSS patients’ 5-year OS. Another retrospective study (35) studied 76 patients with recurrent ESCC patients and found that patients with a PNI >45 had significantly superior OS than those with a PNI ≤45. Further analyses demonstrated that the PNI was an independent factor for OS. In our present study, the optimal cutoff value for PNI calculated using the Cutoff Finder application was 48.15 and CESCC patients with a pretreatment PNI >48.15 had a significantly better 5-year OS than those with a PNI ≤48.15. Moreover, the pretreatment PNI was an independent prognostic factor on multivariable analysis.
In addition to PNI, gender and RT technique were also found to be independent prognostic factors for CESCC in the present study. The prognosis of female CESCC patients was significantly better than that of male patients, which is consistent with the better prognosis of young women who undergo surgical resection of esophageal cancer in another study (36). In the present study, the survival rates of patients who received IMRT or 3D-CRT were superior to those of patients who received conventional 2D-CRT, likely because IMRT involves a lower radiation dose to healthy organs and less treatment-related acute toxicity (37). Moreover, a higher dose of RT (<60 vs. ≥60 Gy) did not have a survival benefit in our study, which is consistent with the findings of the Radiation Therapy Oncology Group 94-05 study (38).
There are several limitations in this study. First, this study was a retrospective study of a small sample treated in a single center. Second, PNI data were collected only at a single time point prior to treatment, and fluctuations in PNI throughout the treatment and follow-up were not fully documented and analyzed. This is notable because PNI can be affected by various pathological conditions during the treatment process and vary with time. Third, due to the lack of data for the side effects of CRT, these factors were not included in the assessment. To prove the validity and accuracy of PNI for predicting the prognosis of CESCC treated with RT and CRT, a multi-center study with a larger sample size is needed.
Conclusions
Pretreatment PNI was found to be a novel nutrition-based prognostic factor in CESCC patients treated with definitive CRT. Measurement of the PNI is reliable, inexpensive, and routine in the pretreatment work-up for CESCC in clinical practice. Preoperative nutritional interventions and/or intensive adjuvant therapy should be considered for patients with low preoperative PNI levels. Thus, PNI measurement will aid the clinical decision-making regarding individualized treatment selection. Larger-scale studies are warranted to validate these findings.
Acknowledgements
The authors thank all patients who participated in the present study.
Funding: This study was supported in part by grants from the Fujian Provincial Health & Family Planning Commission (2016-ZQN-32), the Fujian Provincial Department of Science & Technology (2017Y9079), and the Fujian Provincial Platform for Medical Laboratory Research and Key Laboratory for Tumor Individualized Active Immunity (FYKFKT-2017015).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the Fujian Province Cancer Hospital Institutional Review Board (IRB number: KT2018-016-01). All patients provided written informed consent prior to treatment, and all information was anonymized prior to analysis.
References
- Hoeben A, Polak J, Van De Voorde L, et al. Cervical esophageal cancer: a gap in cancer knowledge. Ann Oncol 2016;27:1664-74. [Crossref] [PubMed]
- Sakanaka K, Ishida Y, Fujii K, et al. Long-term outcome of definitive radiotherapy for cervical esophageal squamous cell carcinoma. Radiat Oncol 2018;13:7. [Crossref] [PubMed]
- Valmasoni M, Pierobon ES, Zanchettin G, et al. Cervical Esophageal Cancer Treatment Strategies: A Cohort Study Appraising the Debated Role of Surgery. Ann Surg Oncol 2018;25:2747-55. [Crossref] [PubMed]
- Prieto I, Montemuiño S, Luna J, et al. The role of immunonutritional support in cancer treatment: Current evidence. Clin Nutr 2017;36:1457-64. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. [Crossref] [PubMed]
- Yoshida T, Kinoshita H, Yoshida K, et al. Prognostic impact of perioperative lymphocyte-monocyte ratio in patients with bladder cancer undergoing radical cystectomy. Tumour Biol 2016;37:10067-74. [Crossref] [PubMed]
- Yodying H, Matsuda A, Miyashita M, et al. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann Surg Oncol 2016;23:646-54. [Crossref] [PubMed]
- Chen L, Yan Y, Zhu L, et al. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag Res 2017;9:849-67. [Crossref] [PubMed]
- Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer 2013;13:158. [Crossref] [PubMed]
- Miao J, Xiao W, Wang L, et al. The value of the Prognostic Nutritional Index (PNI) in predicting outcomes and guiding the treatment strategy of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT) with or without chemotherapy. J Cancer Res Clin Oncol 2017;143:1263-73. [Crossref] [PubMed]
- Ye LL, Oei RW, Kong FF, et al. The prognostic value of preoperative prognostic nutritional index in patients with hypopharyngeal squamous cell carcinoma: a retrospective study. J Transl Med 2018;16:12. [Crossref] [PubMed]
- Okada S, Shimada J, Kato D, et al. Clinical Significance of Prognostic Nutritional Index After Surgical Treatment in Lung Cancer. Ann Thorac Surg 2017;104:296-302. [Crossref] [PubMed]
- Yang Z, Zhang B, Hou L, et al. Pre-operative prognostic nutritional index predicts the outcomes for triple-negative breast cancer. Tumour Biol 2014;35:12165-71. [Crossref] [PubMed]
- Hsieh MC, Rau KM, Chiang PH, et al. Impact of Prognostic Nutritional Index on Overall Survival for Patients with Metastatic Urothelial Carcinoma. J Cancer 2018;9:2466-71. [Crossref] [PubMed]
- Sakurai K, Tamura T, Toyokawa T, et al. Low Preoperative Prognostic Nutritional Index Predicts Poor Survival Post-gastrectomy in Elderly Patients with Gastric Cancer. Ann Surg Oncol 2016;23:3669-76. [Crossref] [PubMed]
- Sun K, Chen S, Xu J, et al. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2014;140:1537-49. [Crossref] [PubMed]
- Shibutani M, Maeda K, Nagahara H, et al. The prognostic significance of the postoperative prognostic nutritional index in patients with colorectal cancer. BMC Cancer 2015;15:521. [Crossref] [PubMed]
- Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol 2019;234:1794-802. [Crossref] [PubMed]
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598-606. [Crossref] [PubMed]
- Miller KR, Bozeman MC. Nutrition therapy issues in esophageal cancer. Curr Gastroenterol Rep 2012;14:356-66. [Crossref] [PubMed]
- Laviano A, Di LL, Koverech A. Nutrition support and clinical outcome in advanced cancer patients. Proc Nutr Soc 2018;77:388-93. [Crossref] [PubMed]
- Kühn T, Sookthai D, Graf ME, et al. Albumin, bilirubin, uric acid and cancer risk: results from a prospective population-based study. Br J Cancer 2017;117:1572-9. [Crossref] [PubMed]
- Wang CY, Hsieh MJ, Chiu YC, et al. Higher serum C-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiother Oncol 2009;92:270-5. [Crossref] [PubMed]
- Suh B, Park S, Shin DW, et al. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann Oncol 2014;25:2260-6. [Crossref] [PubMed]
- Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 2012;23 Suppl 8:viii6-9.
- Kim S, McClave SA, Martindale RG, et al. Hypoalbuminemia and Clinical Outcomes: What is the Mechanism behind the Relationship. Am Surg 2017;83:1220-7. [PubMed]
- Seager RJ, Hajal C, Spill F, et al. Dynamic interplay between tumour, stroma and immune system can drive or prevent tumour progression. Converg Sci Phys Oncol 2017.3. [PubMed]
- Fang P, Jiang W, Davuluri R, et al. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol 2018;128:584-90. [Crossref] [PubMed]
- Davuluri R, Jiang W, Fang P, et al. Lymphocyte Nadir and Esophageal Cancer Survival Outcomes After Chemoradiation Therapy. Int J Radiat Oncol Biol Phys 2017;99:128-35. [Crossref] [PubMed]
- Jin S, Cao S, Xu S, et al. Clinical impact of pretreatment prognostic nutritional index (PNI) in small cell lung cancer patients treated with platinum-based chemotherapy. Clin Respir J 2018;12:2433-40. [Crossref] [PubMed]
- Hirahara N, Tajima Y, Fujii Y, et al. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: a propensity score matching analysis. BMC Cancer 2018;18:285. [Crossref] [PubMed]
- Hirahara N, Tajima Y, Fujii Y, et al. Preoperative Prognostic Nutritional Index Predicts Long-Term Surgical Outcomes in Patients with Esophageal Squamous Cell Carcinoma. World J Surg 2018;42:2199-208. [Crossref] [PubMed]
- Han L, Song Q, Jia Y, et al. The clinical significance of systemic inflammation score in esophageal squamous cell carcinoma. Tumour Biol 2016;37:3081-90. [Crossref] [PubMed]
- Wu N, Pang LW, Chen ZM, et al. Tumour length is an independent prognostic factor of esophageal squamous cell carcinomas. Chin Med J (Engl) 2012;125:4445-8. [PubMed]
- Su XD, Zhang X, Xie HJ, et al. Younger women have a better prognosis among patients with esophageal squamous cell carcinoma after esophagectomy. J Thorac Dis 2016;8:872-9. [Crossref] [PubMed]
- Deng JY, Wang C, Shi XH, et al. Reduced toxicity with three-dimensional conformal radiotherapy or intensity-modulated radiotherapy compared with conventional two-dimensional radiotherapy for esophageal squamous cell carcinoma: a secondary analysis of data from four prospective clinical trials. Dis Esophagus 2016;29:1121-7. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]


