Advances in nanotechnology and asthma
Asthma
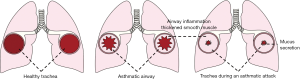
Asthma is one of the most common chronic and non-communicable diseases in children and adults. At present, more than 300 million people are living with asthma worldwide, among whom about 30 million asthmatic patients are in China (1). In recent years, the global asthma prevalence is rising annually. Asthma is a heterogeneous disease in which genetic polymorphisms interact with environmental factors. It can be divided into different phenotypes according to clinical characteristics (e.g., the age of onset and disease severity), triggers (exercise and viral infection), and inflammation types (e.g., eosinophilic, neutrophilic, paucigranulocytic, and mixed granulocytic) (2,3). While no specific treatment has been available for asthma due to its complex pathogenesis, long-term standardized therapies can effectively alleviate symptoms, reduce attacks, and improve the prognosis (Figure 1). Most asthmatic patients respond well to corticosteroid inhalation. The combinations of steroids with bronchodilators such as long- or short-acting beta-receptor agonists (LABA or SABA) or leukotriene receptor antagonists (LTRAs) are considered to be the first-line control strategy for asthma (4). However, asthma control is still poor in some asthmatic patients, even after the use of the maximum dose of corticosteroids (4). Importantly, the expenditure in these patients accounts for more than 60% of asthma-related medical costs (5). In addition to inhaled glucocorticoids, human monoclonal antibodies and cytokine/chemokine antagonists have been used to treat moderate to severe refractory asthma. However, these strategies can only achieve limited success due to the heterogeneity of asthma (6-8).
Nanotechnology
Nanotechnology is a multidisciplinary field of research, which manipulates and controls atoms and molecules sized 0.1–100 nm and manufactured relevant materials or structures (9). As a powerful driving force for the development of biomedicine, nanotechnology has stretched over fields including the early diagnosis, treatment, and prevention of diseases and the bioengineering studies, showing promising prospects. Thousands of nanomaterials have been used in biomedical research. Nanoparticles are one of the most widely studied drug delivery systems, as described in over 25,000 articles in the past decade (10). The Doxil nanotechnology platform was approved in 1995 and has become commercially successful. Since then, many nano-drugs have been approved by the US FDA, and some other drugs are also in clinical development. Nanomedicine is mainly focused on cancer and infectious diseases. For instance, doxorubicin liposomes (11), albumin-bound paclitaxel (12), and pegaspargase (13) have been developed as intravenous anticancer drugs. Other anticancer drugs are so far in phase II and III clinical trials. However, few studies have focused on the design of nanoparticles for the treatment of asthma. Here we will review the literature to highlight some of the recent advances in this field, with an attempt to further explore the potential of nanoparticles in asthma treatment.
Potentials of nanotechnology in asthma treatment
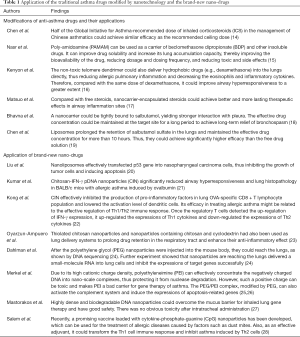
The currently available nano-drugs can be divided into two categories: (I) traditional molecular drugs improved by nanotechnology; and (II) the brand-new nano-drugs. The former is an improvement on traditional medicines, while the latter emphasizes the use of nanomaterials themselves as drugs. Here we will separately elucidate the application of these two categories of nano-drugs in asthma management (Table 1).
Full table
Application of nano-modified anti-asthma drugs
The nano-modification technology of traditional drugs mainly includes the research and development of nano-particle carriers with precise surface patterns, the carrying of existing drugs through drug-targeting reagents, and so on, to achieve targeted drug delivery, reduce toxic and side effects, and improve the solubility of insoluble drugs.
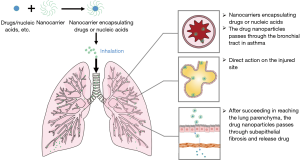
The prevention and treatment of asthma require long-term medication. The traditional anti-asthma drugs are mainly administered intravenously, orally, or by inhalation. The inhaled agents are the mainstream medications to maintain good asthma control, and their sizes are closely related to their bioavailability and efficacy. Compared with systemic drug administration, the targeted drug delivery by inhalation enables the drugs to directly reach the lungs, thus avoiding the first-pass effect and improving bioavailability (Figure 2). Glucocorticoid is the most effective drug to control airway inflammation caused by asthma (29). After a glucocorticoid is inhaled, it has a potent topical anti-inflammatory effect. The drug directly acts on the respiratory tract, which requires less dosage and has fewer systemic adverse reactions. A multi-center clinical trial of fluticasone in the treatment of asthma in China many years ago showed that half of the Global Initiative for Asthma-recommended dose of inhaled corticosteroids (ICS) in the management of Chinese asthmatics achieved similar efficacy as the recommended ceiling dose (14). Although hormone inhalation therapy dramatically reduces the side effects (compared with systemic hormones), long-term high-dose hormone inhalation will inevitably bring some adverse reactions such as inhibition of adrenal axis, oral fungal infection, and osteoporosis. To reduce the side effects of long-term high-dose hormone inhalation and further improve the bioavailability of hormones, PEGylated Poly(amidoamine) (PAMAM) dendrimer, a typical dendrimer, has been widely studied and applied. According to Nasr et al., PAMAM could be used as a carrier of beclomethasone dipropionate (BDP) and other insoluble drugs. It could improve drug solubility and increase its lung accumulation capacity, thereby improving the bioavailability of the drug, reducing dosage and dosing frequency, and reducing toxic and side effects (15). Also, the well-defined non-toxic telomere dendrimer has also been reported to be an efficient nanocarrier with greater loading capacity and better stability than micelles (for more than 6 months) (30). This nanocarrier can also deliver hydrophobic drugs (e.g., dexamethasone) into the lungs directly, thus reducing allergic pulmonary inflammation and decreasing the eosinophils and inflammatory cytokines. Therefore, compared with the same dose of dexamethasone, it can improve airway hyperresponsiveness to a greater extent (16).
At present, the most commonly used drugs for alleviating symptoms included inhaled β2-receptor agonists, anticholinergic drugs, and short-acting theophylline. These drugs can rapidly relieve bronchospasm, usually within a few minutes, and the therapeutic effect can last for several hours. They are the first choice for alleviating acute symptoms in patients with mild to moderate asthma and also can be used for preventing exercise-induced asthma. These drugs should be used on demand, and long-term, excessive use of one single agent should be avoided. Their adverse reactions include skeletal muscle tremor, hypokalemia, and arrhythmia. Also, long-term use of a single LABA was associated with an increased risk of death from asthma (31).
According to Matsuo et al., compared with free steroids, nanocarrier-encapsulated steroids achieved better and more lasting therapeutic effects in airway inflammation sites (17). Also, a nanocarrier can tightly be bound to salbutamol, yielding stronger interaction with pleura. The effective drug concentration could be maintained at the target site for a long period to achieve long-term relief of bronchospasm (18). Based on the above two studies, Chen et al. found that liposomes prolonged the retention of salbutamol sulfate in the lungs and maintained the effective drug concentration for more than 10 hours. Thus, they achieved significantly higher efficacy than the free drug solution (19). Compared with the micronized salbutamol sulfate, the nanoparticles loaded with the same drug were less affected in the human oropharynx and had higher peripheral deposition, which indicated that the nanoparticles had smaller size and greater topical bioavailability and could last for a longer period (32).
While no specific treatment has been available for asthma due to its complex pathogenesis, long-term standardized asthma treatment can effectively control asthma symptoms, prevent asthma attacks, and protect normal lung function. At present, the priority of asthma treatment has also changed from the use of bronchodilators to the treatments for fighting against inflammation and reducing airway responsiveness. Also, gene therapy and molecularly targeted therapy have also become hot research topics.
Application of brand-new nano-drugs
New nano-drugs are highly effective and low-toxicity therapeutic drugs or diagnostic drugs based on new nano-particles by utilizing new nanostructures or nano-characteristics. Since few studies have explored the new nanotherapy of asthma and the attack of asthma is closely related to the abnormal expressions of multiple genes, the effective combination of nano-gene carriers with genes represents a new direction in asthma therapy. Nano-sized gene carriers, with nanoparticles as the gene transfer vectors, wrap DNA and RNA in nanoparticles or adsorb them on the surface of nanoparticles; meanwhile, specific target molecules such as specific ligands and monoclonal antibodies are coupled on the surface of nanoparticles. By binding the target molecules with specific receptors on the surface of cells, the nanoparticles can enter cells via cellular uptake, thus achieving safe and effective targeted drug and gene therapies (Figure 3). Therefore, selecting and constructing appropriate nanocarriers are cutting-edge topics in the gene therapy of asthma.
In recent years, new nano-gene therapy has become an alternative therapy for asthma patients. How to deliver DNA or RNA molecules to the target cells has long been a challenge in gene therapy. Due to the presence of a defense mechanism against foreign genetic material in the human body, free DNA and RNA molecules will be degraded rapidly and thus cannot achieve the desired therapeutic effects. It has been proposed that lipid molecules can be made into nanoparticles for wrapping DNA and RNA molecules. After their sizes and chemical compositions are changed, these nanoparticles can be safely transported into lung tissue. The nanocarrier-based gene delivery system provides a highly adjustable platform for therapeutic gene delivery, but the inability to achieve sustained, high-level gene expression in vivo is another major obstacle (27).
Many non-viral vectors based on liposomes and macromolecule polymers have been developed to convert nucleic acids into nanoparticles for lung delivery (33). Polyethyleneimine (PEI) is one of the most important polymeric gene carriers (34). Applications of nanoparticles such as chitosan (35), polyamidoamine dendrimers (36), and biodegradable poly(lactic-co-glycolic) acid (PLGA) (37) in the delivery of nucleic acids to lungs have also been described.
Nanoliposomes could effectively transfect p53 gene into nasopharyngeal carcinoma cells, thus inhibiting the growth of tumor cells and inducing apoptosis (20). Due to the toxicity of nanoparticles, however, microbubble nanocarriers have also been considered. They are composed of pulmonary surfactants and newly synthesized amphiphilic compounds, which are safe, biocompatible, and biodegradable. Some inventors are also trying to obtain patents of inhaled liposomes and liposome-drug-liposomes in treating lung diseases or systemic diseases or in gene therapy (38). A new solid lipid nanoparticle with a particle size of 50-1000 nm has also shown promising future. With good biocompatibility, it can protect compounds from degradation and can better control drug release (39).
According to Kumar et al., Chitosan-IFN-γ pDNA nanoparticles (CIN) can significantly reduce airway hyperresponsiveness and lung histopathology in BALB/c mice with allergic asthma induced by ovalbumin (21). In another study, they found CIN could effectively inhibit the production of pro-inflammatory factors in lung OVA-specific CD8+ T lymphocyte population and lower the activation level of dendritic cells. Its efficacy in treating allergic asthma may be related to the effective regulation of Th1/Th2 immune response. Once the regulatory T cells detect the up-regulation of IFN-γ expression, it will up-regulate the expressions of Th1 cytokines and down-regulate the expressions of Th2 cytokines (22). Thiolated chitosan nanoparticles and nanoparticles containing chitosan and cyclodextrin have also been used as lung delivery systems to prolong drug retention in the respiratory tract and enhance their anti-inflammatory effect (23).
After the polyethylene glycol (PEG) nanoparticles are injected into the mouse body, they can reach the lungs, as shown by DNA sequencing (24). The further experiment shows that nanoparticles reaching the lungs can successfully deliver a small-molecule RNA into lung cells and inhibit the expressions of target genes (24). Due to its high cationic charge density, polyethyleneimine (PEI) can effectively concentrate the negatively charged DNA into nano-scale complexes, thus protecting it from nuclease degradation. However, such a positive charge can be toxic and makes PEI a bad carrier for gene therapy of asthma. The PEG/PEI complex, modified by PEG, can also activate the complement system and induce the expressions of apoptosis-related genes (25,26). Therefore, we need safer and more effective biodegradable carriers. Mastorakos et al. introduced highly dense and biodegradable DNA nanoparticles that could overcome the mucus barrier for inhaled lung gene therapy and have good safety. There was no obvious toxicity after intratracheal administration (27). Recently, a promising vaccine loaded with cytokine-phosphate-guanine (CpG) nanoparticles has been developed, which can be used for the treatment of allergic diseases caused by factors such as dust mites. Also, as an effective adjuvant, it could transform the Th1 cell immune response and inhibit asthma induced by Th2 cells (28).
To sum up, asthma is a highly complex chronic airway inflammatory disease, involving a variety of cells and cellular components. Therefore, asthma has many potential molecular targets including cytokines, chemokines, transcription factors, tyrosine kinases, and their receptors, as well as costimulatory molecules for gene silencing or over-expression of specific targets, which can be delivered together with drugs through nanoparticles.
Summary and prospect
This article reviews the potential benefits of nanocarrier-based drugs and gene therapy for asthma patients. As a potentially useful technique for the treatment and diagnosis of lung diseases, nanotechnology has become one of the top priority research areas. Pre-clinical studies using nanocarriers have demonstrated the safety and effectiveness of nanocarriers. However, many challenges still need to be overcome before nanotherapy can be applied in clinical practice. A thorough understanding of the mechanism of nanocarriers and the improved chemical structures of these materials are essential for the design and implementation of more reasonable clinical studies.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (NSFC: 81770028) and the Project of Shenzhen Basic Research Plan (JCYJ20170307095633450).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163-96. [Crossref] [PubMed]
- Tarlo SM, Malo JL. ATS/ERS. An ATS/ERS report: 100 key questions and needs in occupational asthma. Eur Respir J 2006;27:607-14. [Crossref] [PubMed]
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012;18:716-25. [Crossref] [PubMed]
- Tan LD, Bratt JM, Godor D, et al. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy 2016;9:71-81. [Crossref] [PubMed]
- Israel E, Reddel HK. Severe and Difficult-to-Treat Asthma in Adults. N Engl J Med 2017;377:965-76. [Crossref] [PubMed]
- Mitchell PD, El-Gammal AI, O'Byrne PM. Emerging monoclonal antibodies as targeted innovative therapeutic approaches to asthma. Clin Pharmacol Ther 2016;99:38-48.. [Crossref] [PubMed]
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo- controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 2013;188:1294-302. [Crossref] [PubMed]
- Barnes PJ. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat Rev Immunol 2018;18:454-66. [Crossref] [PubMed]
- Heidel JD, Davis ME. Clinical Developments in Nanotechnology for Cancer Therapy. Pharm Res 2011;28:187-99. [Crossref] [PubMed]
- Anselmo AC, Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release 2014;190:15-28. [Crossref] [PubMed]
- Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs 1997;54 Suppl 4:15-21. [Crossref] [PubMed]
- Moreno-Aspitia A, Perez EA. Nanoparticle albumin-bound paclitaxel (ABI-007): a newer taxane alternative in breast cancer. Future Oncol 2005;1:755-62. [Crossref] [PubMed]
- Zeidan A, Wang ES, Wetzler M. Pegasparaginase: where do we stand? Expert Opin Biol Ther 2009;9:111-9. [Crossref] [PubMed]
- Chen P, Zhao HT, Sun L, et al. The efficacy of half of the Global Initiative for Asthma recommended dose of inhaled corticosteroids in the management of Chinese asthmatics. Zhonghua Jie He He Hu Xi Za Zhi 2005;28:458-63. [PubMed]
- Nasr M, Najlah M, D'Emanuele A, et al. PAMAM dendrimers as aerosol drug nanocarriers for pulmonary delivery via nebulization. Int J Pharm 2014;461:242-50. [Crossref] [PubMed]
- Kenyon NJ, Bratt JM, Lee J, et al. Self-assembling nanoparticles containing dexamethasone as a novel therapy in allergic airways inflammation. PLoS One 2013;8:e77730. [Crossref] [PubMed]
- Matsuo Y, Ishihara T, Ishizaki J, et al. Effect of betamethasone phosphate loaded polymeric nanoparticles on a murine asthma model. Cell Immunol 2009;260:33-8. [Crossref] [PubMed]
- Bhavna, Ahmad FJ, Mittal G, et al. Nano-salbutamol dry powder inhalation: a new approach for treating broncho-constrictive conditions. Eur J Pharm Biopharm 2009;71:282-91.
- Chen X, Huang W, Wong BC, et al. Liposomes prolong the therapeutic effect of anti-asthmatic medication via pulmonary delivery. Int J Nanomedicine 2012;7:1139-48. [PubMed]
- Liu H, Qin X, Xian J, et al. Nanoliposome-mediated p53 gene therapy for nasopharyngeal carcinoma. Chinese Journal of Otorhinolaryngology-Skull Base Surgery 2008;14:21-4.
- Kumar M, Kong X, Behera AK, et al. Chitosan IFN-gamma-pDNA nanoparticle (CIN) therapy for allergic asthma. Genet Vaccines Ther 2003;1:3. [Crossref] [PubMed]
- Kong X, Hellermann GR, Zhang W, et al. Chitosan interferon gamma nanogene therapy for lung disease: modulation of T-cell and dendritic cell immune responses. Allergy Asthma Clin Immunol 2008;4:95-105. [Crossref] [PubMed]
- Oyarzun-Ampuero FA, Brea J, Loza MI, et al. Chitosan hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma Int J Pharm 2009;381:122-9. [Crossref] [PubMed]
- Dahlman JE, Kauffman KJ, Xing Y, et al. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc Natl Acad Sci U S A 2017;114:2060-5. [Crossref] [PubMed]
- Merkel OM, Beyerle A, Beckmann BM, et al. Polymer-related off target effects in non-viral siRNA delivery. Biomaterials 2011;32:2388-98. [Crossref] [PubMed]
- Merkel OM, Urbanics R, Bedocs P, et al. In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine-graft-poly(ethylene glycol) block copolymers. Biomaterials 2011;32:4936-42. [Crossref] [PubMed]
- Mastorakos P, da Silva AL, Chisholm J, et al. Highly compacted biodegradable DNA nanoparticles capable of overcoming themucus barrier for inhaled lung gene therapy. Proc Natl Acad Sci U S A 2015;112:8720-5. [Crossref] [PubMed]
- Salem AK. A promising CpG adjuvant-loaded nanoparticle-based vaccine for treatment of dust mite allergies. Immunotherapy 2014;6:1161-3. [Crossref] [PubMed]
- Guidelines for the Prevention and Treatment of Bronchial Asthma. (2016 Edition). Chinese Journal of Tuberculosis and Respiratory Diseases 2016;39:241-2. [PubMed]
- Jackson JK, Zhang X, Llewellen S, et al. The characterization of novel polymeric paste formulations for intratumoral delivery. Int J Pharm 2004;270:185-98. [Crossref] [PubMed]
- Weatherall M, Wijesinghe M, Perrin K, et al. Meta-analysis of the risk of mortality with salmeterol and the effect of concomitant inhaled corticosteroid therapy. Thorax 2010;65:39-43. [Crossref] [PubMed]
- Chan JG, Wong J, Zhou QT, et al. Advances in device and formulation technologies for pulmonary drug delivery. AAPS PharmSciTech 2014;15:882-97. [Crossref] [PubMed]
- Di Gioia S, Trapani A, Castellani S, et al. Nanocomplexes for gene therapy of respiratory diseases: targeting and overcoming the mucus barrier. Pulm Pharmacol Ther 2015;34:8-24. [Crossref] [PubMed]
- Merdan T, Callahan J, Petersen H, et al. Pegylated polyethylenimine-Fab′ antibody fragment conjugates for targeted gene delivery to human ovarian carcinoma cells. Bioconjug Chem 2003;14:989-96. [Crossref] [PubMed]
- Köping-Höggård M, Tubulekas I, Guan H, et al. Chitosan as a nonviral gene delivery system. Structure-property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Ther 2001;8:1108-21. [Crossref] [PubMed]
- Rudolph C, Lausier J, Naundorf S, et al. In vivo gene delivery to the lung using polyethylenimine and fractured polyamidoamine dendrimers. J Gene Med 2000;2:269-78. [Crossref] [PubMed]
- Bivas-Benita M, Lin MY, Bal SM, et al. Pulmonary delivery of DNA encoding mycobacterium tuberculosis latency antigen Rv1733c associated to PLGA-PEI nanoparticles enhances T cell responses in a DNA prime/protein boost vaccination regimen in mice. Vaccine 2009;27:4010-7. [Crossref] [PubMed]
- Insmed Incorporated: US9549939 (2017). Available online: >http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&u=%2Fnetahtml%2FPTO%2Fsearch-adv.htm&r=10&f=G&l=50&d=PTXT&p=1&S1=9549939&OS=9549939&RS=9549939
- Wang W, Zhu R, Xie Q, et al. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomedicine 2012;7:3667-77. [Crossref] [PubMed]