Dexmedetomidine versus midazolam and propofol for sedation in critically ill patients: Mining the Medical Information Mart for Intensive Care data
Introduction
Patients in the intensive care units (ICU) are usually treated with many invasive therapies such as mechanical ventilation (MV), which may cause anxiety and discomfort. Actually, nonnegligible factors such as pain, delirium as well as oversedation have an effect on prognosis (1). Therefore, sedation is essential for ICU patients to facilitate tolerance of MV, reduce agitation, enhance comfort and reduce metabolic demands (2).
Midazolam, a gamma-aminobutyric acid (GABA) agonist, is a traditional sedative for ICU patients. Its advantages include that either continuous or intermittent administration are acceptable and a short duration of effect. However, continuously infusion and a high dose of midazolam may increase the risk of delirium and respiratory depression (3,4). Propofol is also a preferred sedative for ICU patients because of its short duration of effect and quick onset. Dexmedetomidine, a highly selective alpha-2 receptor agonist target at the locus coeruleus, provides an alternative to GABA agonist for ICU sedation (5). The benefits of dexmedetomidine for ICU patients are inconclusive. Studies suggest that dexmedetomidine reduced duration of MV compared to other sedatives but did not improve length of hospital stay and length of ICU stay (6,7).
Long stay in the ICU adds to the burden of health care costs and can be considered an indicator for efficiency of health care resource utilization, thus more evidence is needed to guide the sedative medication for ICU patients (8,9). Our study compared dexmedetomidine with propofol and midazolam using data from the Medical Information Mart for Intensive Care III (MIMIC-III) database. The effects of these three drugs on mortality, length of hospital stay, length of ICU stay, duration of MV, and sedation efficiency were explored.
Methods
Clinical database
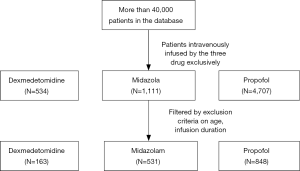
The MIMIC-III database is a large and freely available database comprising more than 40,000 patients in the ICU of the Beth Israel Deaconess Medical Center in Boston, MA, USA between 2001 and 2012. De-identified data including lab results, electronic documentation, bedside monitor trends and waveforms are available (10). This study used a public de-identified database thus informed consent and approval of the Institutional Review Board was waived. Our access to the database was approved after completion of the Collaborative Institutional Training Initiative (CITI program) web-based training course called “Data or Specimens Only research” (Record ID: 22861401).
Data extraction
Subjects who were continuously given dexmedetomidine, midazolam or propofol exclusively as sedative by intravenous infusion for more than 24 hours but less than 100 hours were identified from the database. Patients with other administration routes like intravenous injection and intramuscular injection were excluded. Another exclusion criterium was an age beyond the range of 18 to 90.
Demographic information collected at admission such as age, gender and primary diagnosis was extracted from the database. We simplified the diagnoses by clustering similar primary diagnoses according to the Clinical Classifications Software suggested by Agency for Healthcare Research and Quality (AHRQ) (11). Then we classified the diagnoses into different system categories such as circulation, respiration, renal according to respective organs involved. Mortality and dynamic physiological measurements such as Simplified Acute Physiologic Score (SAPS) II evaluated at ICU admission were also extracted from the MIMIC-III database directly (12). Overall infusion time of each sedative was computed by summing every discontinuous infusion fragment, which was defined as duration between end time and start time. Length of ICU stay was defined as duration between date of discharge or transferring or death and admission date. The duration of ventilation was calculated by counting any ventilator setting and ending records. If a single patient had multiple ventilation records, only those during which sedative was used for more than 6 hours were counted. Patients without ventilation records were also included in the analysis.
Patients on any vasopressor/inotrope (norepinephrine, epinephrine, phenylephrine, vasopressin, dopamine, dobutamine, milrinone) during ICU stay were considered hemodynamically unstable. Similarly, use of neuromuscular blocking agents [NMBA, e.g., (Cis)Atracurium, Rocuronium, Vecuronium, Pancuronium and Atracurium] was also considered a confounding factor for mortality since patients who required them were in more severe conditions and had higher risk of mortality.
Richmond Agitation-Sedation Score (RASS or RAS Score) is a commonly used scoring system to assess the depth of sedation (13). All RASS records of sedated patients were collected from the MIMIC-III database. However, not all sedated patients had RASS due to a considerable amount of missing data, especially patients in the dexmedetomidine group. Thus, only the RASS of midazolam and propofol groups would be compared.
Some patients were admitted to ICU for several times and their records related to each time of admission were included in our study separately in order to make the most of data. These cases were marked as repeated so their influences and possible bias could be under control in further analysis.
Outcomes
The primary outcome was ICU mortality. The secondary outcomes included length of ICU stay and RASS. As a competing risk event, in-ICU death might shorten length of ICU stay. Therefore, competing risk analysis was performed for the comparison of length of ICU stay between each group.
Statistical analysis
Firstly, we summarized the baseline data of included patients, who were grouped by the sedative they used. Features of the three groups were compared to evaluate the data’s heterogeneity. Then, the data was fitted with a logistic regression model to find out independent risk factors for ICU death. Furthermore, stratified analysis was performed according to the risk factors identified in the logistic regression. Box-plots were used to visualize the results of stratified analysis. At last, propensity score matching (PSM) was applied to balance the confounding factors that influenced sedative efficacy and create comparable units of midazolam and propofol patients. Covariates included age, gender, infusion time, SAPS II. Matching ratio was set as 1:1 and nearest neighbor matching method was used. The comparison of RASS between midazolam and propofol groups after PSM were visualized in a box-plot.
Categorical variables were expressed as the number and percentage, and their differences among groups were compared using Chi-squared test. Noncontinuous variables and continuous variables that didn’t follow normal distribution were expressed as median and quartiles, and were analyzed with non-parametric methods (Mann-Whitney-Wilcoxon for two groups, Kruskal-Wallis for multi-groups). Continuous variables that followed normal distribution were expressed as mean and standard deviations, and t-test (two groups) or Analysis of Variance (ANOVA) (multiple groups) was used for these variables. We used postgreSQL 9.5 to construct the MIMIC-III database locally. All analyses were performed with R language 3.4.2. MatchIt package was used for PSM and ggplot2 packages was used for data visualization (14,15). A P value <0.05 was considered statistically significant.
Results
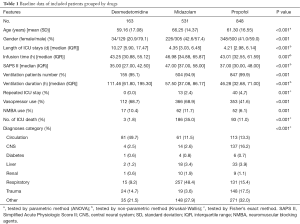
After screening the database according to the inclusion and exclusion criteria, a total of 1,542 unique ICU records were identified in the MIMIC-III database, among which 163 belonged to the dexmedetomidine group and 531 belonged to the midazolam group and 848 for propofol (Figure 1). Median and quartile infusion doses were 0.4 (0.3–0.6) mcg/kg/h for dexmedetomidine, 2.0 (1.0–4.0) mg/h for midazolam and 40 (28–55 mcg/kg/min) for propofol. The baseline information of each group was summarized in Table 1. The three groups were highly heterogeneous in almost every feature.
Full table
Analysis of the data revealed that patients who were given midazolam had the highest ICU mortality (dexmedetomidine: 1.8%, midazolam: 35.0%, propofol: 11.0%, P<0.001). The dexmedetomidine group had the longest length of ICU stay [dexmedetomidine: 10.27 (5.90, 17.47) d, midazolam: 4.35 (3.03, 6.45) d, propofol: 4.21 (2.98, 6.14) d, P<0.001] as well as ventilation duration [dexmedetomidine: 111.46 (61.80, 195.30) h, midazolam: 57.50 (37.08, 86.17) h, propofol: 46.28 (32.68, 71.00) h, P<0.001]. In addition, up to a half of patients with circulation system diseases were given dexmedetomidine as sedative (49.7%). And patients with respiratory diseases were more likely to be given midazolam (48.4%).
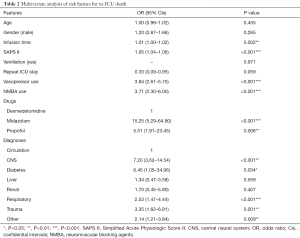
The multivariate analysis of logistic regression show similar results that dexmedetomidine was associated with a lower mortality compared with midazolam (OR 15.25; 95% CI, 5.29–64.80, P<0.001) and propofol (OR 5.51; 95% CI, 1.91–23.45, P=0.006) (Table 2). Besides drugs, infusion time, SAPS II, diagnoses, vasopressor use and NMBA use were also independent risk factors for in-hospital death of the ICU patients. One score elevation in SAPS II would increase the risk of death by 1.05 times (OR 1.05; 95% CI, 1.04–1.06, P<0.001). Similarly, one more hour of sedatives infusion would increase the risk of death by 1.01 times [1.01 (1.00–1.02), P=0.002]. What’s more, patients that used any vasopressor as well as NMBA had a higher risk of death compared with others (OR 3.84; 95% CI, 2.61–5.75) for vasopressor and 3.71 (2.30–6.00) for NMBA, P<0.001 for both). And patients with central neural system (CNS) and respiratory diseases would have an extremely higher risk of death compared with patients with circulation system diseases [7.20 (3.62–14.54) for CNS and 2.53 (1.47–4.44) for respiratory, P<0.001 for both].
Full table
Based on the analyses above, further analysis was performed with patients stratified into several categories according to SAPS II and diagnoses. SAPS II was divided into 4 quarters according to the quartiles (Figures 2,3A). Dexmedetomidine group had the lowest mortality in all four SAPS II quarters. As the SAPS II rose (especially in quarter 3 and quarter 4), patients in the midazolam group had an extremely high mortality rate.
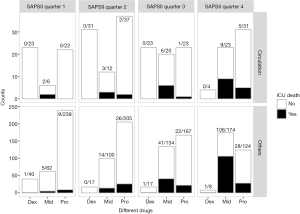
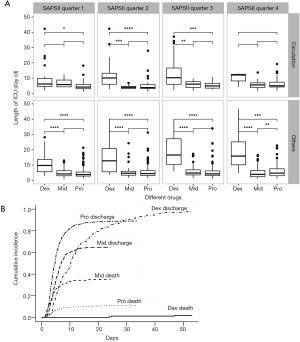
Stratified analyses were also performed in survivors to compare the secondary outcomes. Generally, dexmedetomidine had the longest length of ICU stay in most of the SAPS II quarters and diagnosis groups. Midazolam group and propofol group didn’t show significant differences (Figure 3A). The competing risk analysis suggested that despite the competing effects of in-ICU death, dexmedetomidine still show a longer ICU stay (P<0.001), especially in the first 10 days (Figure 3B).
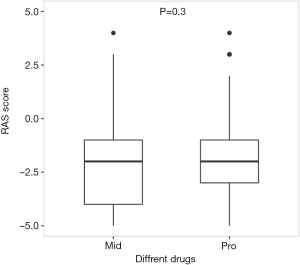
Only 3 patients in dexmedetomidine group had RASS records during sedative infusion. There were few missing data in other groups that 322 patients in midazolam group and 520 patients in propofol group had records of RASS during sedative infusion. Thus, only the sedative efficacy of midazolam and propofol were compared after PSM (Table 3, Figure 4). PSM created compared units of midazolam and propofol groups where potential confounding factor such as age, gender, infusion duration and SAPS II were homogeneous and sedative efficacy became comparable. And the result showed that there was no significant difference in sedative efficacy between midazolam and propofol (P=0.300) (Figure 5).

Full table

Discussion
Our study revealed that dexmedetomidine was significantly associated with lower mortality and increased length of ICU stay compared to propofol and midazolam. Moreover, ICU patients with propofol and midazolam infusions might achieve a similar depth of sedation level with a similar RAS score.
Mortality in the dexmedetomidine group was lower than that in the propofol and midazolam group, which was supported by both univariate and multivariate analyses. The findings of our study lacked strong convincing power because of the limitations of retrospective design. However, our conclusions were supported by other studies (16,17). The decreased mortality of dexmedetomidine group may be attributed to the following factors. Unlike midazolam and propofol, dexmedetomidine provides arousable sedation, in which patients can cooperate and communicate with healthcare providers and avoid over sedation (5). And it is reported that dexmedetomidine improved the sleep quality and modified sleep patterns in critically ill patients (18).
Our study revealed that midazolam was associated with high mortality and most of death cases had high SAPS II, which indicated a more severe illness. In cases that SAPS II was higher than 52 (Quarter 4), the mortality of midazolam group exceeded 50%. Similar results were reported in previous studies. A multicenter ICU database analysis including more than 3,000 patients reported that continuous midazolam infusion was associated with a higher mortality (risk ratio 1.32) and lower likelihood of earlier ICU discharge than propofol (19).
Although its benefits on mortality was confirmed in our study, dexmedetomidine unexpectedly increased that length of ICU stay compared with midazolam and propofol. Since death might shorten the length of ICU stay, known as a competing effect, the competing risk analysis was performed. Nevertheless, the results still suggested that patients using dexmedetomidine stayed longer in ICU. Several factors might exert complex impact on length of ICU stay, such as nutritional status and family support, which were not considered in our study because of missing or unquantifiable data (20). In addition, choice effects of medical decider might also have an unignorable contribution. Some studies reported that dexmedetomidine might reduce the economic costs (21,22). Therefore, Patients who had a long stay in hospital might be more likely to use dexmedetomidine to reduce costs. However, other studies had results opposite to our study. A meta-analysis done by Chen et al. revealed that dexmedetomidine reduced the length of ICU stay by 14% (95% CI, 1% to 24%; five studies, 1,223 participants, very low-quality evidence) (23). But there were also studies supporting our results. In an analysis carried out in an academic medical center, dexmedetomidine use was associated with increased lengths of ICU and hospital stay (21). These disparities in results indicate that more qualified research is needed.
The SAPS II is a method to evaluate the severity of the patients’ illness. The characters considered in this scoring system include age, vital signs, admission type, and multi-organ functions. In our logistic regression, SAPS II showed van extremely strong relation with ICU mortality (OR 1.05; 95% CI, 1.04–1.06, P<0.001), indicating its powerful ability to predict outcomes. However, the association between age and mortality lacked statistical significance, which could be explained by the collinearity with SAPS II.
The dexmedetomidine patients had few records of RASS, which might attribute to the light and arousable sedation of dexmedetomidine. With a longer time at target sedation, dexmedetomidine was found to be noninferior to midazolam and propofol in light sedation (RASS 0 to −3) in a random trial done by Jakob et al. (6). However, Jakob et al. found that the time at target sedation significantly decreased in dexmedetomidine patients when deep sedation was required (RASS −4 to −5) in their pilot study (7). Due to the limited data size, we did not analyze the sedation efficacy of dexmedetomidine and focused on other two forceful sedatives, midazolam and propofol. PSM is a powerful method to compare unbalanced groups. In our study, we chose age, gender, SAPS II and infusion time as confounding factors to perform the matching. And we found a similar sedative efficacy between these two sedatives, as other researches did (24,25).
In this retrospective study, we analyzed data from the MIMIC-III, a database containing information about tens of thousands of ICU patients, and applied PSM methods to reduce bias. Some limitations exist in our study. Firstly, the retrospective study revealed an association rather than causal relationship. Secondly, the missing data in RASS made it impossible to evaluate sedation efficacy of dexmedetomidine. At last, many factors inevitably introduced bias into the analysis of length of ICU stays, which might complicate the analysis and made the results inconclusive.
Conclusions
Dexmedetomidine was significantly related to lower mortality when compared with midazolam and propofol. However, its benefits on length of ICU stay was unproven. At the same time, midazolam had a relatively higher mortality than propofol and dexmedetomidine in patients with high SAPS II. Propofol and midazolam had similar sedative efficacy. Further evaluation is needed.
Acknowledgements
The datasets generated and/or analysed during the current study are available in the MIMIC-III v1.4, (https://mimic.physionet.org). The authors appreciate the efforts of the Medical Information Mart for Intensive Care III Database. Thanks to the researchers at the MIT Laboratory for Computational Physiology and collaborating research groups.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med 2014;370:444-54. [Crossref] [PubMed]
- Sydow M, Neumann P. Sedation for the critically ill. Intensive Care Med 1999;25:634-6. [Crossref] [PubMed]
- Forster A, Gardaz JP, Suter PM, et al. Respiratory depression by midazolam and diazepam. Anesthesiology 1980;53:494-7. [Crossref] [PubMed]
- Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med 2015;41:2130-7. [Crossref] [PubMed]
- Gertler R, Brown HC, Mitchell DH, et al. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13-21. [Crossref] [PubMed]
- Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA 2012;307:1151-60. [Crossref] [PubMed]
- Ruokonen E, Parviainen I, Jakob SM, et al. Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med 2009;35:282-90. [Crossref] [PubMed]
- González-Cortés R, López-Herce-Cid J, García-Figueruelo A, et al. Prolonged stay in pediatric intensive care units: mortality and healthcare resource consumption. Med Intensiva 2011;35:417-23. [PubMed]
- Mamoli A, Censori B, Casto L, et al. An analysis of the costs of ischemic stroke in an Italian stroke unit. Neurology 1999;53:112-6. [Crossref] [PubMed]
- Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3:160035. [Crossref] [PubMed]
- Cowen ME, Dusseau DJ, Toth BG, et al. Casemix adjustment of managed care claims data using the clinical classification for health policy research method. Med Care 1998;36:1108-13. [Crossref] [PubMed]
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957-63. [Crossref] [PubMed]
- Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338-44. [Crossref] [PubMed]
- Ho DE, Imai K, King G, et al. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software 2011;42:1-28. [Crossref]
- Wickham H. Ggplot2: elegant graphics for data analysis. Use R! New York: Springer, 2009.
- Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation 2013;127:1576-84. [Crossref] [PubMed]
- Ji F, Li Z, Young N, et al. Perioperative dexmedetomidine improves mortality in patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth 2014;28:267-73. [Crossref] [PubMed]
- Alexopoulou C, Kondili E, Diamantaki E, et al. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology 2014;121:801-7. [Crossref] [PubMed]
- Lonardo NW, Mone MC, Nirula R, et al. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. Am J Respir Crit Care Med 2014;189:1383-94. [Crossref] [PubMed]
- Awad A, Bader-El-Den M, McNicholas J. Patient length of stay and mortality prediction: A survey. Health Serv Manage Res 2017;30:105-20. [Crossref] [PubMed]
- Patanwala AE, Erstad BL. Comparison of Dexmedetomidine Versus Propofol on Hospital Costs and Length of Stay. J Intensive Care Med 2016;31:466-70. [Crossref] [PubMed]
- Turunen H, Jakob SM, Ruokonen E, et al. Dexmedetomidine versus standard care sedation with propofol or midazolam in intensive care: an economic evaluation. Crit Care 2015;19:67. [Crossref] [PubMed]
- Chen K, Lu Z, Xin YC, et al. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev 2015;1:CD010269. [PubMed]
- Guinter JR, Kristeller JL. Prolonged infusions of dexmedetomidine in critically ill patients. Am J Health Syst Pharm 2010;67:1246-53. [Crossref] [PubMed]
- McKeage K, Perry CM. Propofol: a review of its use in intensive care sedation of adults. CNS Drugs 2003;17:235-72. [Crossref] [PubMed]