
The relationship between preliminary efficacy and prognosis after first-line EGFR tyrosine kinase inhibitor (EGFR-TKI) treatment of advanced non-small cell lung cancer
Introduction
Lung cancer is a malignant tumor with the highest morbidity and mortality both worldwide and within China. According to the 2015 statistics (1), there were a total of 733,000 new cases of lung cancer in China, and the total number of deaths was about 610,000 (male: 432,400, female: 177,800). NSCLC accounts for 85–90% of all diagnosed lung cancers. About 70% of patients are at an advanced stage when initially diagnosed. For patients with advanced non-small cell lung cancer (NSCLC), the efficacy of traditional platinum-based chemotherapy and radiotherapy has plateaued; thus, the overall survival (OS) of most patients is not satisfactory (2).
In recent years, molecular-targeted therapy for lung cancer driver genes has progressed rapidly. The proportion of driver mutations in the EGFR tyrosine kinase region is 15–40%, which is particularly prevalent in Asians, females, and non-smokers or mild smokers with NSCLC (3-6). More than 90% of known EGFR driver mutations are 19del or 21L858R (7,8). With the ongoing series of large-scale clinical trials (9-11), recent data have revealed that first-line targeted therapy for advanced NSCLC with EGFR-sensitive mutation shows a higher response rate and a longer PFS compared with chemotherapy, and the median PFS is about 10–12 months. Thus, EGFR tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib and erlotinib have been widely used for first-line treatment, while in China icotinib has been mainly approved for clinical use. Clinically, we have observed that some patients have rapid tumor shrinkage at the initial stage of first-line EGFR-TKI treatment, but some patients have slower tumor shrinkage. It remains unknown whether different preliminary efficacy is related to PFS. In our study, we aimed to analyze the relationship between preliminary efficacy (tumor shrinkage within 1 month) and PFS after first-line EGFR-TKI treatment.
Methods
Patients
We identified and reviewed the clinical data of patients who were diagnosed with NSCLC at Shanghai Chest Hospital from January 2013 to January 2017. The study protocol was approved by the Ethics Committee of Shanghai Chest Hospital [KS(Y)1708] and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). Due to the retrospective nature of this study, the need for informed consent was waived. The inclusion criteria were as follows: (I) patients with stage IIIb/IV NSCLC (NSCLC staging was performed according to the 7th edition of the TNM classification); (II) patients whose tumors were positive for EGFR-sensitive mutation such as exon 19 deletion or 21L858R point mutation [the Amplification Refractory Mutation System (ARMS) was used to detect mutations in the EGFR gene]; (III) patients who had responded to first-line EGFR-TKI treatment without the resistance mutation. The baseline clinical characteristics included age at diagnosis, tumor histology, smoking status, gender, etc.
Clinical assessments
Patients were given 150 mg of erlotinib daily or 250 mg of gefitinib daily, while another group of patients who were treated with icotinib received 125 mg three times daily. Clinical follow-up exams included a physical examination, an imaging examination, and laboratory tests, which were performed every 4 weeks. Efficacy was evaluated every month according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1), including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). We defined the response of the first month as preliminary efficacy. Patients who achieved PR within 1 month and SD (−30% to 0) within 1 month were analyzed in our study. If first-line treatment failed, second-line treatment would be given. All patients were regularly followed up after receiving targeted therapy in Shanghai Chest Hospital. The clinical data were complete and traceable. The follow-up ended on February 5th, 2018. The PFS was calculated from the date of initiation of EGFR-TKIs to the date of disease progression or the last follow-up visit.
ARMS method
DNA was extracted from five serial slices of a 5-µm paraffin section using the DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). PCR was performed, and the results were analyzed according to the manufacturer’s protocol of the DxS EGFR mutation test kit (DxS) (12).
Statistical analysis
SPSS22.0 statistical software (IBM, Armonk, NY, USA) was used for data processing. PFS was analyzed with the Kaplan-Meier method. Single factor analysis and Cox multivariate regression analysis were used to explore the significant factors of survival. A P value of <0.05 was considered as statistically significant.
Results
Patient characteristics
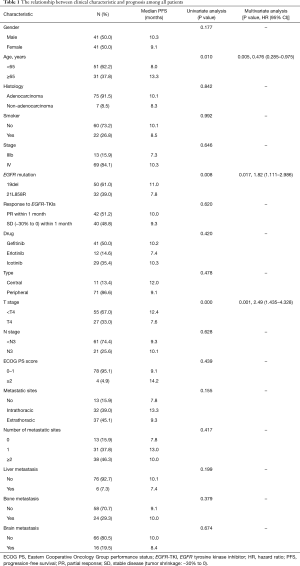
A total of 82 patients with EGFR-TKI-sensitive advanced NSCLC confirmed by histopathology from January 2013 to January 2017 were retrospectively analyzed. Seventy-five (91.5%) patients were adenocarcinoma, and 7 (8.5%) patients were non-adenocarcinoma. Patients underwent EGFR mutation test by ARMS method, and 50 (61.0%) patients had EGFR 19del mutation while 32 (39.0%) patients had EGFR 21L858R mutation. Forty-two (51.2%) patients achieved PR within 1 month, and 40 (48.8%) patients achieved SD (−30% to 0) within 1 month. Demographic data of all patients are shown in Table 1.
Full table
Progression-free survival (PFS)
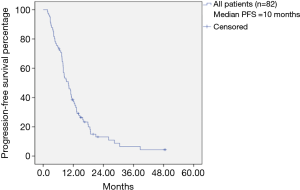
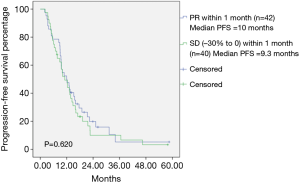
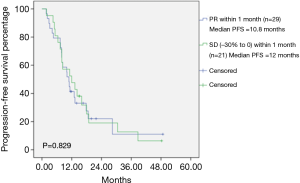
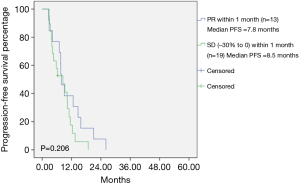
The median PFS among all patients was 10 months (Figure 1). Among the total patients, the median PFS in patients achieving PR within 1 month was 10.0 months, and the median PFS in patients achieving SD (−30% to 0) within 1 month was 9.3 months (Table 1). There was no statistically significant difference between PR within 1 month and SD (−30% to 0) within 1 month (P=0.620) (Figure 2). In the EGFR 19del mutation subgroup, the median PFS in patients achieving PR within 1 month and achieving SD (−30% to 0) within 1 month was 10.8 and 12.0 months, respectively. A statistically significant difference was not found (P=0.829) (Figure 3). In the EGFR 21L858R mutation subgroup, the median PFS in patients achieving PR within 1 month and achieving SD (−30% to 0) within 1 month was 7.8 and 8.5 months, respectively. A statistically significant difference was also not found (P=0.206) (Figure 4).
Univariate and multivariate analysis
Univariate and multivariate analysis of first-line EGFR-TKI treatment showed that age, EGFR mutation type, and T staging had effects on PFS. Patients who were more than 65 years old, had EGFR 19del mutation, along with a T staging less than 4, had a longer PFS; these differences were statistically significant. Liver metastasis, bone metastasis, and brain metastasis were not shown to be related to PFS (Table 1).
Discussion
For EGFR-TKI-sensitive advanced NSCLC, first-line EGFR-TKI treatment has been established as the standard therapy. According to a study, patients who achieved PR/CR after EGFR-TKI treatment had a significantly longer survival than those achieved SD (13). However, the definition of SD according to RECIST criteria is too broad to predict prognosis. It can only be broadly divided into tumor enlargement of less than 20% and tumor regression of less than 30%. Another study evaluated the correlation between efficacy of targeted therapy and prognosis in previously treated advanced NSCLC; longer PFS and OS have been observed in SD (−30% to 0) patients when compared with SD (0 to +20%) patients (14). Clinically, we observed that some patients have rapid tumor shrinkage at the initial stage of first-line EGFR-TKI treatment, but some patients have slower tumor shrinkage. It remains unknown whether different preliminary efficacy is related to PFS. Our retrospective analysis found that there was no significant difference in PFS between PR within 1 month and SD (−30% to 0) within 1 month. This suggests that slow and rapid tumor shrinkage at the initial stage of first-line EGFR-TKI treatment could achieve a similar benefit to PFS. A study showed that for patients with EGFR mutation who achieved CR/PR after EGFR-TKI treatment, the median time to this efficacy was 4.2 weeks (95% CI: 3.9–4.5 weeks), and time to response was not related to PFS or OS in such patients (13). In another similar study, the PFS showed no significant difference in patients who achieved PR with or without early response (15). Some investigators reported that not only could the responsive patients obtain survival benefit, but those SD patients with tumor regression could as well (14). Another study examined individual patient-level data from five randomized trials (EURTAC, IPASS, ENSURE, LUX-Lung 3, and LUX-Lung 6) to assess whether the depth of response at 6 or 12 weeks could be used as a surrogate for PFS or OS, and the conclusion was that it could not (16). These results may support our conclusion, but these studies included patients with previous chemotherapy, and it remains unclear whether chemotherapy has effects on the response or PFS of EGFR-TKI treatment. The sample size is not very large in our study, which may be a limitation.
In the relationship between clinical characteristics and prognosis of total patients, we found that age, EGFR mutation type, and T staging had effects on PFS after first-line EGFR-TKI treatment. A similar retrospective study showed no significant correlation between age and PFS (17). However, a significant benefit was found among patients aged more than 65 years old in our study. This maybe due to the fact that elderly patients have lower metabolic rates and slower tumor growth than younger patients. For different mutation subtypes, it has been previously reported that EGFR 19del mutation has a better prognosis than EGFR 21L858R mutation (18), which was confirmed in our study.
According to the IPASS and EURTAC trial (9,10), patients with EGFR mutation received gefitinib and erlotinib respectively, compared with chemotherapy, the median PFS was 9.5 and 9.7 months. The ICOGEN trial shows that icotinib is not inferior to gefitinib in improving PFS (11). These findings also support the conclusion that there is no significant difference in PFS among these three drugs.
Regarding metastasis, studies have shown that patients with liver metastasis have a significantly shorter PFS after targeted therapy than those without liver metastasis (19), which may be due to the dual blood supply of the liver. However, we did not find this difference because of the small number of patients with liver metastasis. Similarly, bone metastasis and brain metastasis were not shown to be related to PFS, which may be related to simultaneous receiving of targeted therapy and local treatment.
In conclusion, for patients with EGFR-TKI-sensitive advanced NSCLC, there is no correlation between preliminary efficacy (tumor shrinkage within 1 month) and PFS after first-line EGFR-TKI treatment. Whether this is related to OS will be reported in the subsequent analysis, because the follow-up data are not yet mature.
Acknowledgements
We would like to thank all of the investigators for their involvement in this study.
Funding: This work was supported by the Western Medicine Guide Project of Shanghai Committee of Science and Technology (grant No. 16411964700).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Ethics Committee of Shanghai Chest Hospital [KS(Y)1708] and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008).
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Sacher AG, Le LW, Lau A, et al. Real-world chemotherapy treatment patterns in metastatic non-small cell lung cancer: Are patients undertreated? Cancer 2015;121:2562-9. [Crossref] [PubMed]
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther 2014;14:1391-406. [Crossref] [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Tartarone A, Lerose R. Clinical approaches to treat patients with non-small cell lung cancer and epidermal growth factor receptor tyrosine kinase inhibitor acquired resistance. Ther Adv Respir Dis 2015;9:242-50. [Crossref] [PubMed]
- Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol 2009;10:432-3. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 2013;14:953-61. [Crossref] [PubMed]
- Zhang Q, Zhu L, Zhang J. Epidermal growth factor receptor gene mutation status in pure squamous-cell lung cancer in Chinese patients. BMC Cancer 2015;15:88. [Crossref] [PubMed]
- Takeda M, Okamoto I, Nakagawa K. Survival outcome assessed according to tumor response and shrinkage pattern in patients with EGFR mutation-positive non-small-cell lung cancer treated with gefitinib or erlotinib. J Thorac Oncol 2014;9:200-4. [Crossref] [PubMed]
- Zhang J, Huang Y, Li X, et al. The impact of tumor size change after target therapy on survival: analysis of patients enrolled onto three clinical trials of advanced NSCLC from one institution. Onco Targets Ther 2012;5:349-55. [Crossref] [PubMed]
- Chang JW, Hou MM, Hsieh JJ, et al. Early radiographic response to epidermal growth factor receptor-tyrosine kinase inhibitor in non-small cell lung cancer patients with epidermal growth factor receptor mutations: A prospective study. Biomed J 2015;38:221-8. [Crossref] [PubMed]
- Lee CK, Lord S, Marschner I, et al. The Value of Early Depth of Response in Predicting Long-Term Outcome in EGFR-Mutant Lung Cancer. J Thorac Oncol 2018;13:792-800. [Crossref] [PubMed]
- Xu Y, Chen L, Tian Q, et al. Application of epidermal growth factor receptor tyrosine kinase inhibitor as the first-line therapy in patients with advanced non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2010;13:48-53. [PubMed]
- Hung MS, Fang YH, Lin YC, et al. Survival-associated factors of first-line EGFR-tyrosine kinase inhibitor responders and non-responders in lung adenocarcinoma patients with common EGFR mutations. Mol Clin Oncol 2018;8:421-8. [PubMed]
- Jiang T, Cheng R, Zhang G, et al. Characterization of Liver Metastasis and Its Effect on Targeted Therapy in EGFR-mutant NSCLC: A Multicenter Study. Clin Lung Cancer 2017;18:631-639.e2. [Crossref] [PubMed]


