
Acute exacerbation of interstitial lung disease after radical surgery for lung cancer: a case report
Introduction
With the improvement of perioperative treatment and nursing techniques, the incidence of complications and associated mortality in thoracic surgery have gradually decreased. However, there are still serious surgical complications, such as bronchial fistula and pulmonary embolism (1). Currently, interstitial lung disease (ILD) is relatively common in clinical practice, and the condition of some ILD patients may deteriorate after surgery, or even endanger life. Recently, we managed a patient with acute exacerbation of interstitial lung disease (AE-ILD) shown by high-resolution computed tomography (HRCT) of the thorax after radical surgery for lung cancer; the patient fared poorly after treatment with limited relief. Herein, we report the case.
Case presentation
A 69-year-old male patient, in previous good health, was admitted to the local hospital because of cough, expectoration and chest pain for over a month, with no smoking history, no occupational or environmental exposures, and no significant family history. HRCT of the thorax on that day showed an occupying lesion in the left upper lobe and a nodule in the left upper lobe bronchi. Mild interstitial changes were revealed bilaterally (Figure 1A,B). The endoscopic bronchoscopy showed a fungating neoplasm approximately 1 centimeter from the ostium of the left upper inferior lobar bronchus. Tissue biopsy led to the diagnosis of squamous cell lung cancer, T2N1M0, stage IIIA. Preoperative pulmonary function tests showed mild limited ventilation dysfunction and moderate diffusion dysfunction (FEV1: 1.91 L; FVC: 2.66 L; DLCO: 4.56 mmol/min/kPa; DLCO/VA: 1.18 mmol/min/kPa). No obvious abnormality was found in the routine preoperative blood examination.
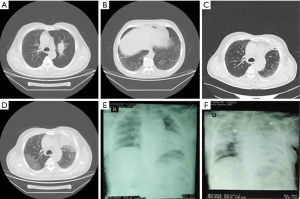
With a diagnosis of lung cancer, open thoracotomy with a left upper lobectomy and lymph node dissection was performed. The patient underwent endotracheal intubation for approximately three and a half hours, in accordance with the duration of the surgical procedure, and was extubated at the end of the surgery. The volume of infused fluids was not mentioned in the surgical record. The patient received intravenous infusions of ceftizoxime (3 g, bid) postoperatively for 8 days and 1,000 mL of additional intravenous fluids for 10 days. HRCT of the thorax on the third postoperative day showed subpleural reticular abnormality (Figure 1C), and the patient was discharged without complaint of discomfort. On the 12th postoperative day, the patient started to have fever accompanied by cough and chest congestion. Arterial blood gas analysis indicated a marked decrease in the partial pressure of oxygen. Sputum analysis revealed no pathogenic evidence of pulmonary infection. HRCT of the thorax on the 14th postoperative day revealed new-onset ground-glass opacities on the background of previous pulmonary fibrosis, evident in both right and left upper lobes, consistent with the characteristic changes of acute exacerbation of ILD (Figure 1D). After readmission, the patient received three cycles of steroid pulse therapy [i.e., (I) methylprednisolone 500 mg qd for 9 days; (II) methylprednisolone 500 mg bid for 4 days; (III) methylprednisolone 250 mg bid for 6 days] along with antibiotic (moxifloxacin, piperacillin-sulbactam and ceftizoxime) and antifungal (voriconazole) drug therapy, though respiratory failure gradually worsened. A chest X-ray performed on the 20th postoperative day and 1 month after the surgery showed that the infiltrative shadows had extended to almost all fields of the lung (Figure 1E,F). Finally, the patient died due to respiratory failure.
Discussion
It is well known that AE-ILD is characterized by deteriorating interstitial inflammation, accompanied by acutely worsening respiratory failure (2). According to the features of the preoperative HRCT of the thorax, especially regarding the interstitial changes in the peripheral zone, this male patient was diagnosed with ILD, and AE-ILD developed following the surgery. Lung surgery has been proven to be associated with considerable mortality in these patients (3-5).
This male patient was considered preoperatively to be in the early period of idiopathic pulmonary fibrosis (IPF) on the basis of the HRCT of the thorax, which was consistent with features of IPF, in combination with his age, sex, general condition, previous medical history and pulmonary function test results. However, lung biopsy was lacking to confirm this diagnosis. The findings regarding AE-ILD almost evolved from IPF, the most common form of ILD in which AE occurs, and extended to the other presentations (6). AE-IPF always occurs spontaneously without verifiable causes. The incidence of postoperative AE-IPF was reported to be fairly high in patients with lung cancer, ranging from 9% to 24% (4,7,8). Potential etiologic factors included, at the least, but are not limited to the following: preoperative disease hyperactivity, high concentrations of oxygen during the operation, mechanical ventilation, intraoperative mechanical-ventilation-related lung injury, and postoperative reduction of the glucocorticoid dose and other drugs (9). Therefore, it is difficult to identify the real cause of AE following surgery. It has been reported that even with relatively low invasion, lung biopsy may also cause AE-IPF (10,11), but the research findings are inconsistent and still need to be confirmed by further studies. Clearly, IPF patients are highly sensitive and respond with irritability to invasive lung manipulation, which is consistent with the pathophysiologic characteristics of IPF.
The prognosis of IPF patients with acute exacerbation is poor. Some studies reported that up to 40% of IPF patients died due to acute exacerbation (12), and the median survival time was approximately 3–4 months (13). Another study found that the mortality associated with AE-IPF exceeded 50% during hospitalization. Among the survivors, the mortality was over 60% within six months post-discharge (14). In terms of drug therapy, there are still no drugs specifically targeted for the treatment of AE-IPF. High-dose steroid pulse therapy has been extensively used in clinical practice, but this treatment is given only a weak recommendation with a low evidence grade in the guidelines (15,16). However, some observational studies have shown that pirfenidone can reduce the incidence of AE-IPF to some extent (17-19). In terms of supportive treatment, considering the hypersensitivity of ILD/IPF patients to chest surgery, preoperative assessment of disease activity and lung function is indispensable, along with appropriate intraoperative general anesthesia and respiratory management (9). In addition, a net-negative fluid balance may benefit the outcome of the patients with AE-ILD by improving oxygenation (20).
In conclusion, it is important for clinicians to be aware of the risk of AE in patients with ILD following lung surgery and the associated high mortality. Surgical indications should be carefully evaluated. If surgery is inevitable, comprehensive lung-protective measures are required intraoperatively, and perioperative use of drugs (pirfenidone, etc.) is also necessary.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Okamoto T, Gotoh M, Masuya D, et al. Clinical analysis of interstitial pneumonia after surgery for lung cancer. Jpn J Thorac Cardiovasc Surg 2004;52:323-9. [Crossref] [PubMed]
- Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636-43. [Crossref] [PubMed]
- Saito Y, Kawai Y, Takahashi N, et al. Survival after surgery for pathologic stage IA non-small cell lung cancer associated with idiopathic pulmonary fibrosis. Ann Thorac Surg 2011;92:1812-7. [Crossref] [PubMed]
- Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604-11.e3. [Crossref] [PubMed]
- Watanabe A, Kawaharada N, Higami T. Postoperative Acute Exacerbation of IPF after Lung Resection for Primary Lung Cancer. Pulm Med 2011;2011:960316. [Crossref] [PubMed]
- Azadeh N, Moua T, Baqir M, et al. Treatment of acute exacerbations of interstitial lung disease. Expert Rev Respir Med 2018;12:309-13. [Crossref] [PubMed]
- Yano T, Koga T, Ninomiya S, et al. A review of Japanese literatures concerning surgery for lung cancer with idiopathic interstitial pneumonia. Kyobu Geka 2002;55:131-3; discussion 133-4. [PubMed]
- Sato S, Shimizu Y, Goto T, et al. Survival after repeated surgery for lung cancer with idiopathic pulmonary fibrosis: a retrospective study. BMC Pulm Med 2018;18:134. [Crossref] [PubMed]
- Sakamoto S, Homma S, Mun M, et al. Acute exacerbation of idiopathic interstitial pneumonia following lung surgery in 3 of 68 consecutive patients: a retrospective study. Intern Med 2011;50:77-85. [Crossref] [PubMed]
- Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J 2001;17:175-9. [Crossref] [PubMed]
- Kondoh Y, Taniguchi H, Kitaichi M, et al. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med 2006;100:1753-9. [Crossref] [PubMed]
- Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 2014;190:773-9. [Crossref] [PubMed]
- Spagnolo P, Wuyts W. Acute exacerbations of interstitial lung disease: lessons from idiopathic pulmonary fibrosis. Curr Opin Pulm Med 2017;23:411-7. [Crossref] [PubMed]
- Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356-63. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Homma S, Bando M, Azuma A, et al. Japanese guideline for the treatment of idiopathic pulmonary fibrosis. Respir Investig 2018;56:268-91. [Crossref] [PubMed]
- Iwata T, Yoshino I, Yoshida S, et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study). Respir Res 2016;17:90. [Crossref] [PubMed]
- Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040-7. [Crossref] [PubMed]
- Iwata T, Yoshida S, Nagato K, et al. Experience with perioperative pirfenidone for lung cancer surgery in patients with idiopathic pulmonary fibrosis. Surg Today 2015;45:1263-70. [Crossref] [PubMed]
- Mizuno Y, Iwata H, Shirahashi K, et al. The importance of intraoperative fluid balance for the prevention of postoperative acute exacerbation of idiopathic pulmonary fibrosis after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2012;41:e161-5. [Crossref] [PubMed]


