
Obese patients have higher risk of breast cancer-related lymphedema than overweight patients after breast cancer: a meta-analysis
Introduction
Lymphedema (LE) is a substantial problem in women after breast cancer. It has been reported to affect 10% to 64% of breast cancer survivors (1). LE after breast cancer is featured by regional swelling and typical in one arm, the pathogenesis of which is excess accumulation of protein-rich fluid in interstitial space (2). Depending on the extent of edema, symptoms of LE include pain, heaviness/fullness, arm tightness, limb dysfunction and poor quality of life (3,4).
Current understanding of risk factors can inform LE prevention and management strategies. Nonetheless, treatment-related risk factors are largely not qualifiable, because they are generally dictated by the type and stage of disease and available treatment options. Body mass index (BMI) is one of the risk factors for LE (5). More than 50 percent of breast cancer patients are overweight or obese (6). This meta-analysis reports what the effects of BMI using cut-point to distinguish obese from overweight patients (<25, 25–29.9, ≥30 kg/m2 for normal weight, overweight and obesity, respectively) on LE (7).
Methods
Search strategy
We systematically searched the main English-language databases, including PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL), and the main Chinese databases, including China National Knowledge Infrastructure (CNKI) and WanFang Data from inception through June 1, 2018 in humans. The following keywords and/or MeSH terms were used: [“breast cancer” or “breast carcinoma” or “mammary cancer” or “breast tumor”, [lymphedema or breast cancer related lymphedema (BCRL), or “arm edema”] and [“body mass index”, “body weight”, overweight, obesity or obese].
Inclusion and exclusion criteria
The included studies met the following six criteria: (I) published research articles; (II) female patients with or over 18 years old; (III) primary unilateral breast cancer and LE was defined as ipsilateral upper swelling; (IV) articles that stratified BMI as normal weight (BMI <25 or <24 kg/m2), overweight (BMI <25–29.9 or 24–28 kg/m2) and obesity (BMI ≥30 or ≥28 kg/m2); (V) articles were written in English or Chinese; (VI) we accepted the study with the largest sample size when the authors published several studies in the same subjects. Exclusion criteria: (I) review, meta-analysis, editorial or comment papers, and case reports; (II) articles that studied breast benign tumor, bilateral breast cancer, primary lymphedema, or metastatic disease; (III) articles that evaluated the effect of BMI change on LE; (IV) articles that measured LE within 3 months of diagnosis or surgery because arm-related changes during this timeframe were considered as potentially indicative of an acute treatment related response.
Data extraction
Two authors selected articles independently. In case of disagreement with each other, it was resolved through careful reexamination and discussed with a third author to reach a consensus according to the predetermined inclusion criteria. The collected information was as follows: surname of first author, year of publication, type of study, country, sample size, definition and measurement methods of LE, follow up time, and the number of LE and non-LE patients in different BMI levels.
Quality evaluation
The quality of the eligible studies was assessed by two authors independently according to the Newcastle Ottawa Scale (NOS) (8). The NOS is a tool used to assess the quality of non-randomized studies that includes eight items categorized into three sections: selection, comparability and clinical outcome (cohort study) or exposure (case-control study). A study can be rewarded a maximum of one star for each numbered item within the selection and clinical outcome or exposure categories. A maximum of two stars can be given for comparability. NOS score ranges from 0 to 9 and with a score of ≥7 indicating high quality.
Statistical analysis
The datum was summarized using Excel 2007. The results of the meta-analysis were calculated using Stata 11. We calculated odds ratios (OR) with 95% confidence intervals (95% CI) by random effects model to estimate the relationship between different BMI levels and LE. We tested the heterogeneity of the studies using a Q test and the I2 value. I2>50% and P<0.05 indicated significant heterogeneity (9,10). A subgroup analysis was performed to investigate sources of heterogeneity when I2>50%. A sensitivity analysis was performed by omitting each study in sequence to evaluate the effect of a single study on the overall estimate. Begg’s funnel plots and Egger’s test were employed to explore the potential publication bias (11).
Results
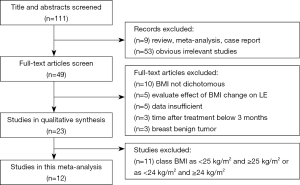
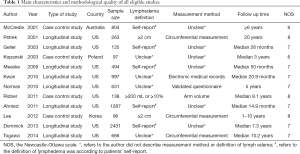
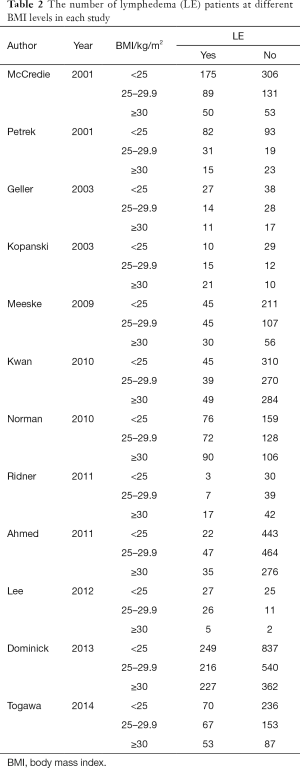
We identify 111 potentially relevant articles, 12 of which meet the inclusion criteria (12-23). The flow chart of the selection process is shown in Figure 1. The characteristics and methodology of the included studies are summarized in Table 1. The number of LE patients at different BMI levels in each study is summarized in Table 2. Eight articles are longitudinal study, and four articles are case control study. Nine studies are from the United States (U.S), and the others are from Australia, Korea and Poland. The largest sample size is 2,431, and the smallest is 96. The most common method of LE measurement is the arm circumference, which define LE as a circumferential measurement difference ≥2 cm between arms. The follow up time is between median 14.9 months to 20 years after breast cancer among the 12 studies. Twelve selected studies include 8,039, 3,548, 2,570, 1,921 breast cancer survivors and 2,102, 831, 668, 603 cases of ipsilateral arm LE in overall patients, normal weight patients, overweight patients and obese patients, respectively. The incidence of LE is 26.15%, 23.42%, 25.99%, 31.39% in overall patients, normal weight patients, overweight patients and obese patients, respectively.
Full table
Full table
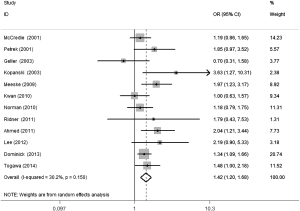
The meta-analysis results reveal significant difference that the OR is 1.42 (95% CI, 1.20 to 1.68) for the BMI 25–30 versus BMI <25 kg/m2 group, 1.39 (95% CI, 1.21 to 1.60) for the BMI ≥30 versus BMI 25–30 kg/m2 group, and 1.84 (95% CI, 1.47 to 2.32) for the BMI ≥30 versus BMI <25 kg/m2 group (Figures 2-4). There was no heterogeneity in the BMI 25–30 versus BMI <25 kg/m2 comparison (I2=30.2%, P=0.150) and in the BMI ≥30 versus BMI 25–30 kg/m2 comparison (I2=0.6%, P=0.438). However, heterogeneity was in the BMI ≥30 versus BMI <25 kg/m2 comparison (I2=53.0%, P=0.015). Furthermore, the OR is 2.83 (95% CI, 1.37 to 5.88) for the case control study subgroup, 1.72 (95% CI, 1.33 to 2.21) for the longitudinal study subgroup and 1.76 (95% CI, 1.41 to 2.19) for the United State subgroup in the BMI ≥30 versus BMI <25 kg/m2 group by subgroup analysis (Figures 5,6).
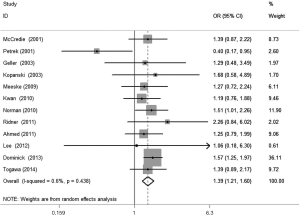
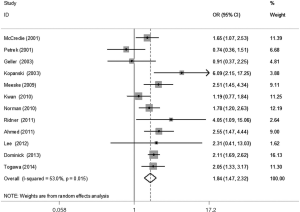
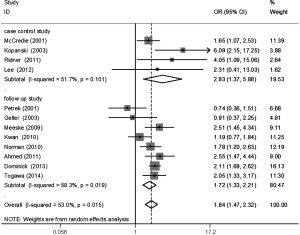
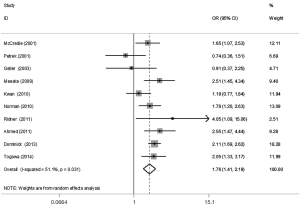
A sensitivity analysis was used to estimate the impact of each individual study on the accumulated OR by omitting individual studies one at a time. The results show that no individual study significantly affects the accumulated OR, which displays statistically stable results (data not shown). We used Begg’s funnel plots and Egger’s test to estimate the potential publication bias of the included literature. The shapes of the Begg’s funnel plots do not show any obvious asymmetry, and Egger’s test also do not display strong statistical evidence for publication bias (data not shown), indicating that the combined results are reliable.
Discussion
Our findings suggest that BMI is a risk factor for LE, which is similar to reports from two previous meta-analyses (24,25). The two previous meta-analyses did not distinguish overweight and obesity, but we did. Our study provides additional insight for LE. First, incidence of LE after breast cancer shows an upward trend with the increase of BMI levels. Second, the OR is 1.42 for the overweight versus normal weight group, 1.39 for the obese versus overweight group, and 1.84 for the obese versus normal weight group, which suggests a positive association between weight and LE. Moreover, one previous study suggested that the degree of lymphedema was related positively to the level of obesity (26). So, it is necessary and significant to distinguish obesity from overweight patients, because obese patients are more likely to suffer from LE than overweight patients. Third, the OR is 1.76 for obese versus normal weight patients in U.S. subgroup, which is slight lower than the worldwide level. Fourth, whether if in case control study subgroup or in longitudinal study subgroup, the result shows that the effect of obesity on LE may not disturb by study type.
The relation between obesity and LE is complex. A functional link has emerged between lymphatic malfunction and the pathogenesis of obesity. Possibly, people with higher BMI need greater blood circulation and lymphatic system to facilitate fluid flow. It is likely to result in the capacity of lymph and circulatory imbalanced (27). Is it the outcome of a heavier arm with more subcutaneous tissue, adipose, and skin, regarding as a cistern for lymphatic fluid, or is it because of the operation needing to be more extensive as a product of the presence of adipose tissue and therefore more destructive to the lymphatics (28,29). Someone also pointed out that the obese patients are susceptible to fat necrosis, poor wound healing and infection, obesity reduced muscle-pumping efficiency within loose tissues, the separation of deep lymphatic channels by additional subcutaneous fat, and excess body weight may limit the effectiveness of elastic compression, thus leading to LE (30).
The results of Begg’s funnel plots and Egger’s test show this study has better stability and smaller publication bias. So, it can offer evidence and guidelines to prevent and treat lymphedema in clinical work. Recently, Duyur Cakit et al. report that obesity deteriorates the complex decongestive therapy efficacy (31).
However, BMI is just one of the risk factors for LE, and there are other known risk factors. The well-established risk factors contain regional lymph node radiation and axillary lymph node dissection (28). A 5-year cohort study showed that participants with more weight gain, lymph node metastases, and larger circumferential difference between arms are more likelihood of developing persistent LE (32). These risk factors alone do not accurately predict who will develop arm lymphedema and who will not. Wang et al. declared that their scoring system containing the level of axillary lymph node dissection, history of hypertension, surgery on dominant arm, radiotherapy, and surgical infection/seroma/early edema can be a simple and easy tool for physicians to estimate the risk of LE (33). Whether the potential contribution of the cancer itself or genetic predisposition would be a risk factor for LE is little known. Findings from human beings and animal models provide preliminary evidence for a contribution of genetic susceptibility to the development of secondary LE after breast cancer (34). Further studies are needed to improve our understanding of risk factors, as well as prevention and treatment strategies.
There are some limitations associated with our results that should be noted. First, retrospective studies may encounter recall or selection bias, which possibly influencing the reliability of our results. Second, we did not pay much attention to other potential factors that might have influenced our results, even if all included studies were collected carefully with similar inclusion criteria. Thus, it would be better to have a randomized controlled study with a large sample size.
In a word, this meta-analysis provides strong evidence that obese patients have higher risk of LE than overweight patients after breast cancer. So, it is necessary and significant to distinguish overweight from obese patients. The physicians should pay more attention to obese patients after breast cancer. Our results will generate awareness of LE, which remains one of the most common and distressing complications for breast cancer survivors.
Acknowledgements
Funding: This study was supported by a National Natural Science Foundation of China grant (No. 81471459) to H Zhang.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer 2012;118:2237-49. [Crossref] [PubMed]
- Hespe GE, Nores GG, Huang JJ, et al. Pathophysiology of lymphedema-Is there a chance for medication treatment? J Surg Oncol 2017;115:96-8. [Crossref] [PubMed]
- Chowdhry M, Rozen WM, Griffiths M. Lymphatic mapping and preoperative imaging in the management of post-mastectomy lymphoedema. Gland Surg 2016;5:187-96. [PubMed]
- De Vrieze T, Gebruers N, Devoogdt N. Concerning Quality of Life Questionnaires in Breast Cancer-Related Lymphedema Patients: Review of the Literature by Cornelissen et al. Lymphat Res Biol 2018;16:421-2. [Crossref] [PubMed]
- Fu MR, Axelrod D, Guth AA, et al. Patterns of Obesity and Lymph Fluid Level during the First Year of Breast Cancer Treatment: A Prospective Study. J Pers Med 2015;5:326-40. [Crossref] [PubMed]
- Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer 2012;118:2277-87. [Crossref] [PubMed]
- WHO Consultation on Obesity (1999: Geneva, Switzerland) & World Health Organization. (2000). Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva: World Health Organization. Available online: .http://www.who.int/iris/handle/10665/42330
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics 2005;21:3672-3. [Crossref] [PubMed]
- Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ 2008;336:1413-5. [Crossref] [PubMed]
- Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676-80. [Crossref] [PubMed]
- McCredie MR, Dite GS, Porter L, et al. Prevalence of self-reported arm morbidity following treatment for breast cancer in the Australian Breast Cancer Family Study. Breast 2001;10:515-22. [Crossref] [PubMed]
- Petrek JA, Senie RT, Peters M, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001;92:1368-77. [Crossref] [PubMed]
- Geller BM, Vacek PM, O'Brien P, et al. Factors associated with arm swelling after breast cancer surgery. J Womens Health (Larchmt) 2003;12:921-30. [Crossref] [PubMed]
- Kopanski Z, Wojewoda T, Wojewoda A, et al. Influence of some anthropometric parameters on the risk of development of distal complications after mastectomy carried out because of breast carcinoma. Am J Hum Biol 2003;15:433-9. [Crossref] [PubMed]
- Meeske KA, Sullivan-Halley J, Smith AW, et al. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Res Treat 2009;113:383-91. [Crossref] [PubMed]
- Kwan ML, Darbinian J, Schmitz KH, et al. Risk Factors for Lymphedema in a Prospective Breast Cancer Survivorship Study The Pathways Study. Arch Surg 2010;145:1055-63. [Crossref] [PubMed]
- Norman SA, Localio AR, Kallan MJ, et al. Risk Factors for Lymphedema after Breast Cancer Treatment. Cancer Epidemiol Biomarkers Prev 2010;19:2734-46. [Crossref] [PubMed]
- Ahmed RL, Schmitz KH, Prizment AE, et al. Risk factors for lymphedema in breast cancer survivors, the Iowa Women's Health Study. Breast Cancer Res Treat 2011;130:981-91. [Crossref] [PubMed]
- Ridner SH, Dietrich MS, Stewart BR, et al. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer 2011;19:853-57. [Crossref] [PubMed]
- Lee SH, Min YS, Park HY, et al. Health-Related Quality of Life in Breast Cancer Patients with Lymphedema Who Survived More than One Year after Surgery. J Breast Cancer 2012;15:449-53. [Crossref] [PubMed]
- Dominick SA, Madlensky L, Natarajan L, et al. Risk factors associated with breast cancer-related lymphedema in the WHEL Study. J Cancer Surviv 2013;7:115-23. [Crossref] [PubMed]
- Togawa K, Ma HY, Sullivan-Halley J, et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Res 2014;16:414. [PubMed]
- Zhu YQ, Xie YH, Liu FH, et al. Systemic analysis on risk factors for breast cancer related lymphedema. Asian Pac J Cancer Prev 2014;15:6535-41. [Crossref] [PubMed]
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. [Crossref] [PubMed]
- Segerström K, Bjerle P, Graffman S, et al. Factors that influence the incidence of brachial oedema after treatment of breast cancer. Scand J Plast Reconstr Surg Hand Surg 1992;26:223-7. [Crossref] [PubMed]
- Mak SS, Yeo W, Lee YM, et al. Predictors of lymphedema in patients with breast cancer undergoing axillary lymph node dissection in Hong Kong. Nurs Res 2008;57:416-25. [Crossref] [PubMed]
- Gillespie TC, Sayegh HE, Brunelle CL, et al. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg 2018;7:379-403. [Crossref] [PubMed]
- Greene AK, Maclellan RA. Obesity-induced Upper Extremity Lymphedema. Plast Reconstr Surg Glob Open 2013;1:e59. [Crossref] [PubMed]
- Arngrim N, Simonsen L, Holst JJ, et al. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: a possible link between obesity and local tissue inflammation? Int J Obes (Lond) 2013;37:748-50. [Crossref] [PubMed]
- Duyur Cakıt B, Pervane Vural S, Ayhan FF. Complex Decongestive Therapy in Breast Cancer-Related Lymphedema: Does Obesity Affect the Outcome Negatively? Lymphat Res Biol 2019;17:45-50. [Crossref] [PubMed]
- Penn IW, Chang YC, Chuang E, et al. Risk factors and prediction model for persistent breast-cancer-related lymphedema: a 5-year cohort study. Support Care Cancer 2019;27:991-1000. [Crossref] [PubMed]
- Wang L, Li HP, Liu AN, et al. A Scoring System to Predict Arm Lymphedema Risk for Individual Chinese Breast Cancer Patients. Breast Care (Basel) 2016;11:52-6. [Crossref] [PubMed]
- Newman B, Lose F, Kedda MA, et al. Possible genetic predisposition to lymphedema after breast cancer. Lymphat Res Biol 2012;10:2-13. [Crossref] [PubMed]


