Diagnosis and management of peripheral lung nodule
Introduction
Solitary pulmonary nodule (SPN) is defined as a single well circumscribed radiographic opacity, up to 30 mm in diameter, surrounded by unaltered aerated lung with no associated atelectasis, hilar enlargement or pleural effusion (1,2). Lesions larger than 30 mm in diameter are called lung masses and are usually considered malignant (3).
In recent years, there has been an increase in the number of lung nodules found on imaging, both incidentally and as part of lung cancer screening programs. Incidental pulmonary nodules are found on 0.1–0.2% of routine chest radiographs (4,5) and on 13% of non-screening chest CT scans (6). In a high-risk smoker population such as the national lung cancer screening trial, the incidence increases to 9% on chest radiographs and 33% using low dose CT scan (7,8). Most of the identified SPN are benign. A final diagnosis of malignancy is obtained in 1–12% (9-15).
Lung cancer is the leading cause of cancer death in the world (16). The 5-year survival rate of patients with lung cancer drops from 82% for stage IA to 6% for stage IV. Accordingly, timely diagnosis of lung cancer at an early stage is of essence since it results in the highest cure rate (17). It is important for the physician to evaluate the clinical and radiological risk factors to identify nodules at high risk for malignancy, thus warranting further evaluation, while avoiding unnecessary procedures for lower risk nodules.
Clinical evaluation
The focus of the clinical evaluation is to determine the presence of risk factors for malignancy and to evaluate the presence of non-malignant conditions that are associated with SPN.
Patients with SPN are usually asymptomatic. When symptoms are present, they usually reflect the underlying condition that resulted in the development of the lung nodule. In the setting of malignancy, the presence of symptoms may represent advanced metastatic disease. Risk factors such as smoking, advanced age, prior history of malignancy, interstitial lung disease, emphysema, and exposure to asbestos, uranium, and radon are associated with a higher chance of a malignant SPN (18-24).
A detailed travel history to areas with high prevalence of mycosis and tuberculosis should be obtained to rule out benign infectious etiology of SPN (25,26). In addition, autoimmune conditions such as rheumatoid arthritis and granulomatosis polyangiitis are frequently associated with pulmonary nodules and should be included in the differential diagnosis of SPN (27).
Radiographic imaging
Chest plain film
Many SPNs are now detected on CT scans but some are still seen first on chest X-ray. It is important to review prior imaging when available to determine any change in the SPN (28). A nodule that is highly calcified or has been stable in size for more than 2 years when compared to previous radiographs has a high likelihood of being benign (29). Technical innovations including bone-suppression software programs have been suggested to improve the sensitivity of chest X-ray for depicting nodules. Frequently, an SPN require further radiological evaluation (30).
CT scan
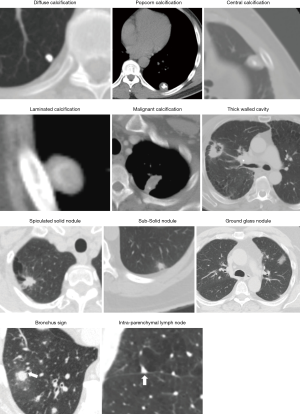
For accurate characterization of small nodules, reconstructed thin-section (≤1.5 mm) CT images should be obtained as it decreases the effect of volume averaging. Measurements should be expressed to the nearest whole millimeter. Routine acquisition of coronal and sagittal series is recommended as it facilitates the distinction between nodules and scars (28). Specific morphologic features such as size, attenuation, location, borders, characteristics, calcification, morphological pattern, and nodule enhancement may be helpful to differentiate benign from malignant disease (Figure 1).
Size
The likelihood of malignancy rises with the increase in the diameter of a nodule. Nodules <5 mm in diameter have a <1% chance of malignancy. The risk increases to 6–28% for nodules between 5–10 mm and to 33–60% for lesions >10 mm (29). In the NELSON screening study, nodules smaller than 5 mm had a 0.4% chance of being malignant which was not considered different from the risk of malignancy in a patient without nodules (30). Recent guidelines have modified the minimal size for determining the need for monitoring up to 5 mm for the BTS guidelines (6) and 6 mm for the Fleischner society guidelines (28).
Attenuation
Nodule attenuation allows the classification of the SPN into solid or sub-solid nodules. Sub-solid nodules are further divided into pure ground-glass nodules (GGN) with no solid component and partly-solid (PSN) with areas of soft-tissue attenuation interspersed with areas of ground-glass attenuation (31). Solid nodules are homogeneous and dense. Sub-solid nodules contain a portion of ground-glass attenuation that is higher than that of normal lung parenchyma and lower than that of soft tissue such that airways and vessels can be visualized through them. They may result from infection, inflammation, hemorrhage, or neoplasm. Those associated with infection may resolve quickly. Persistent sub-solid nodules are more likely to be malignant, specifically primary lung adenocarcinoma (32). To note that in a screened population, Henschke et al. found that sub-solid nodules were more likely to be malignant than a solid one, even when nodule size is taken into account (33).
Location
Upper lobes location of SPN is considered an independent risk factor for malignancy (34). This could be due to a higher concentration of inhaled carcinogens in the upper lobes resulting from cigarette smoking (35).
Border characteristics
A spiculated margin, often described as sunburst or corona radiata sign is associated with the highest risk of malignancy (36). It has a positive predictive value of up to 90%. Some benign conditions such as lipoid pneumonia, focal atelectasis, tuberculoma, and progressive massive fibrosis, may also have a spiculated margin (35,37).
Well-defined smooth or polygonal margins are typically seen in benign nodules but up to one-third may be malignant (2). A lobulated margin has an intermediate risk for malignancy (36).
Calcifications
Common benign patterns of calcification include diffuse solid calcifications, central, lamellar, and popcorn. Diffuse, central, and lamellar patterns are typically seen in granulomatous infections whereas popcorn calcifications are seen in hamartomas. Calcifications pattern such as stippled or eccentric have been associated with malignancy (36).
Morphologic patterns
Fat attenuation between −40 to −120 Hounsfield unit (HU) is suggestive of a hamartoma. It may be seen in metastases, liposarcoma, renal cell cancer and lipoid pneumonia (38).
Cavitation occurs in both infectious and inflammatory conditions as well as malignant SPNs such as squamous cell carcinoma. Wall thickness is a helpful marker. Smooth, thin walls are typically seen in benign lesions, whereas thick, irregular walls are seen in malignant lesions. It has been reported that 95% of cavitary nodules with a wall thickness greater than 15 mm are malignant, and 92% of cavitary nodules with a wall thickness less than 5 mm are benign. A cavity wall thickness of 5–15 mm is not reliable to differentiate benign versus malignant nodules (39,40).
Nodule enhancement
SPNs that enhance more than 20 HU after the injection of intravenous contrast material are usually malignant, whereas enhancement of less than 15 HU suggests benign etiology. This technique is not helpful for nodules smaller than 5 mm as they have a higher likelihood of benignity (35).
Growth rate
Growth is an important factor to differentiate benign and malignant lesions. Growth is assessed by the volume doubling time (VDT). Since nodules are spherical structures, the volume is calculated using the equation 4πr3. Therefore, a 26% increase in the diameter results in doubling of the volume (41,42). In the NELSON screening trial, the risk of malignancy was 0.8%, 4% and 9.9% for a VDT of >600 days, 400–600 days and <400 days respectively (43). Malignant, solid SPNs usually have a VDT of 20–400 days with the majority having VDT <100 days (42). A VDT <20 days is usually reflective of an infectious process (44). Revel et al. reported a negative predictive value for malignancy of 98% when the VDT exceeded 500 days (45). For pure GGO and PSN, a longer VDT of 813±375 and 457±260 has been suggested to document stability (46). The BTS guidelines have incorporated the VDT as part of the management of lung nodules that are 6 mm or larger (6).
Intraparenchymal lymph node (IPN)
IPN, also known as perifissural nodules (PFN), are common causes of benign SPN. On CT imaging, they have sharp borders with oval, rounded, lentiform or triangular shape. They are located below the level of the carina, within 15 mm of the fissure or the pleura. The typical IPNs have contact with interlobar septum. Atypical IPNs are nodules that either meet all above features except being attached to a visible fissure or are attached to a fissure but are convex on one side only (47,48). IPN represent dilated lymphatic channels (49). Large studies looking at long term follow up of patient of more than 4 years suggested that IPN often show larger size and can have interval growth on follow up imaging but are not malignant (50,51). Fleischner society guidelines do not recommend follow-up CT IPN, even if the average dimension exceeds 6 mm (28).
Positron emission tomography (PET)
PET is a recognized imaging modality with a capability of differentiating malignant from normal tissue based on glucose metabolism. Metabolic activity can be measured using the standardized uptake value (SUV). A high SUV indicates increased FDG uptake due to high metabolic glycolytic activity and suggests malignancy or infection/inflammation (48).
Integrated PET/CT is superior to either modality alone (52,53). Therefore, PET scan nowadays is rarely performed without a concurrent CT. In a retrospective study including nodules 7–30 mm, the sensitivity for CT, PET, and PET/CT was 93%, 69%, and 97%, respectively (54). Specificity was 31%, 85% and 85% for the 3 modalities respectively. False negative findings on PET are mainly seen in tumors with low metabolic activity (adenocarcinoma in situ and carcinoid tumors), small tumors (<7–10 mm), and hyperglycemia. False positive results are often secondary to an infectious or inflammatory process (3,55). In a high-risk SPN, a negative PET/CT does not reliably exclude malignancy, and a surgical or non-surgical biopsy may still be needed. Finally, Fleischner society 2017 guidelines recommend PET/CT (along 3-month CT follow-up or biopsy) for the evaluation of SPN >8 mm regardless of pretest risk evaluation (28).
Models to estimate pretest probability of malignancy
An individualized approach is essential when an SPN is found. The selection and interpretation of subsequent tests highly depend on the SPN pretest probability of malignancy. Several factors including patients’ age, smoking status, SPN characteristics (size, location, attenuation, and spiculation), family history of lung cancer or personal history of extra-thoracic malignancy, play a role in determining the pretest probability of malignancy (3,6,19,34,56).
Multiple quantitative models have been developed to help with the calculation of the pretest probability of malignancy (Figure 2). The most familiar and validated ones include the Mayo Clinic (34), the Veterans Affair (19), and the Brock University (56) models. There is no clear evidence that any model is superior to the others. Hence, the characteristics of the targeted population should preferably guide the selection of the predictive model (3). For example, Brock University model was developed and validated based on cohorts of high-risk patients enrolled in lung cancer screening programs (smokers, and former smokers) (56). Applying this model to non-smoker patients with SPNs may lead to overestimation of their risk of malignancy. The Mayo Clinic model may better predict the risk of malignancy in the general population with incidentally found SPNs (34). The inclusion of PET imaging (Herder model) (57) or nodule volume (58) to the Mayo Clinic risk calculator improves its predictive value.
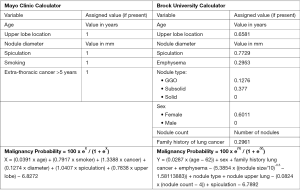
The accuracy of most predictive models appears to be similar (59,60) if not inferior (61) to that of expert clinicians. Therefore, their additive value continues to be challenged. The British Thoracic Society (BTS) incorporated the use of predictive models (Brock and Herder) in its 2015 guidelines (6). On the other hand, the 2013 American College of Chest Physicians (ACCP) guidelines recommend estimating pretest probability for solid nodules >8 mm, but do not advise for or against using predictor tools (62). Based on these models, the probability of malignancy is usually classified into low (<5%), intermediate (5–65%) and high (>65%) (3).
Management
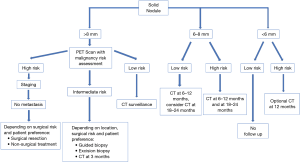
The Fleischner society and the BTS guidelines are the most updated and accepted guidelines for diagnosis and management of incidental pulmonary nodules management (6,28). It is to be noted that the Fleischner guidelines are not applicable for patients less than 35 year old, immunocompromised patients, or those who are already diagnosed with cancer (28). In contrast, BTS guidelines do not exclude nodules in patients with current or previously treated malignancy (6). Both guidelines recommend not to offer follow-up or further investigation for nodules with benign patterns of calcification (diffuse, central, laminated or popcorn), macroscopic fat or typical perifissural/intrapulmonary lymph node. Prior imaging studies should always be reviewed whenever available to determine possible growth or stability (6,28). These guidelines have separate recommendations for solid and sub-solid nodules and are based on nodule size and cancer risk of the patient (Figures 3,4).
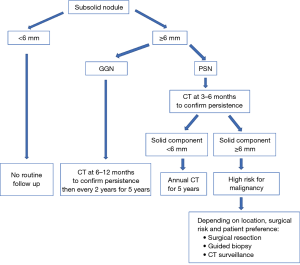
Ground glass nodule (GGN)
GGN <6 mm
No routine follow-up is recommended. An optional 2–4 years follow up can be considered depending on suspicious morphology and risk factors. This recommendation comes from the Asian population data, which shows that up to 10% of such nodules can grow in size and nearly 1% may progress to adenocarcinoma over many years (64).
GGN ≥6 mm
Follow up scanning is recommended initially at 6–12 months, then every 2 years for 5 years (after initial 6–12 months CT). An average of 3–4 years is usually required to establish growth (65,66).
Part solid nodule (PSN)
The amount of solid component is an indicator of aggressive behavior and invasive features. Nodules with solid components <6 mm represent either adenocarcinoma in situ or minimally invasive adenocarcinoma. Nodules with a solid component ≥6 mm have a substantial higher risk of invasiveness and metastasis and require closer follow-up (28,67).
PSN <6 mm
No routine follow up is recommended.
PSN ≥6 mm
For solitary PSN ≥6 mm, initial CT is recommended at 3–6 months to confirm persistence. When the solid component is <6 mm and the PSN is unchanged, a yearly follow up for a minimum of 5 years is recommended. PSNs with a solid component ≥6 mm after an initial follow-up are highly suspicious of invasive malignancy (68). For nodules with suspicious morphology, growing solid component or a solid component >8 mm, further testing with PET/CT, biopsy or surgical resection are recommended (28).
Multiple sub-solid nodules
In case of multiple sub-solid nodules, if the largest nodule is less than 6 mm, infectious causes are most likely and a CT at 3–6 months should be considered. If lesions remain persistent after an initial follow-up scan at 3–6 months, consider follow-up at 2 and 4 years. When at least one of the multiple sub-solid nodules is >6 mm, a CT at 3–6 months should be considered and subsequent management should be based on the most suspicious nodule(s).
Solid nodule
Solid nodule <6 mm
According to Fleishner guidelines, single solid nodules smaller than 6 mm (5 mm or smaller) do not require routine follow-up in low-risk patients. In case of a solid nodule <6 mm but with high-risk factors, there is an option to follow up with a CT in 12 months. An early follow up before 12 months is not recommended in high-risk nodules <6 mm as they rarely advance in stage.
Solid nodule 6–8 mm
Solitary non-calcified solid nodules measuring 6–8 mm in patients with low clinical risk are recommended to undergo initial follow-up at 6–12 months depending on size, morphology, and patient preference. One follow up study is sufficient in most of the cases, but if the stability of the node is uncertain or morphology is suspicious, a second follow up at 18–24 months can be obtained. High-risk 6–8 mm nodules should be followed with two follow-up studies at 6–12 months and again at 18–24 months.
Solid nodule >8 mm
There is a strong recommendation about early follow up with CT scan at 3 months, PET/CT, tissue biopsy or a combination of these modalities. The use of a validated model to estimate the pretest probability of malignancy can help in guiding the management of these patients. Depending on patient preference, that management could be a follow-up in low-risk patients (<5% annual risk), surgical resection in high risk (>65% annual risk) operable patients, or to perform additional test such as PET/CT and/or tissue sampling in patients who are considered to have an intermediate risk (6–65%) of malignancy (69).
Non-surgical biopsy
CT-guided transthoracic biopsy
CT guided transthoracic needle biopsy (TTNB) is one of the non-surgical modalities available to establish the etiology of a suspicious lung nodule. Done under CT guidance, its accuracy depends on several factors, including the size of the nodule, the number of passes and the presence of on-site pathologist. Another factor is the size of the needle used, which could be important if one suspects a lymphoma or a benign etiology. Studies looking into the value of TTNB or aspiration in establishing the diagnosis of peripheral bronchogenic carcinoma found a sensitivity of 90% (CI: 88–91%) and a specificity of 97% (CI: 96–98%) (62). While the rate of false positive is rare (average of 1%), there is a substantial false negative rate (average of 22%). Therefore, in the absence of a malignant result, a benign diagnosis is reassuring; however, a non-diagnostic result should not be used to rule out malignancy. In such cases, other diagnostic modalities, such as surgery, may be needed.
The main complications associated with TTNB include the risk of pneumothorax, pulmonary hemorrhage, infection, and rarely death. Wiener et al. found a low risk of hemorrhage (1%), but a risk of pneumothorax of 15% (70). However, most pneumothoraces were not substantial, with 6.6% of the patients developing a pneumothorax that necessitated a chest tube insertion. Risk factors for pneumothorax post TTNB include age, smoking, COPD, deeper location, small nodule size, number of needle passes and the need to traverse a fissure (3,70).
Conventional bronchoscopy
In the AQuIRE Registry study, bronchoscopy was diagnostic in 312/581 (53%) of peripheral lesions. The diagnostic yield for the transbronchial biopsy (TBB), needle aspiration, brushing and bronchoalveolar lavage was 43%, 47%, 38%, and 19% respectively (71). The yield of bronchoscopy is affected by the size and location of the lung nodule. A low sensitivity of 34% has been reported for peripheral lung lesions <2 cm in size, compared to 63% for lesions >2 cm (62).
Role of fluoroscopic guidance
Baaklini et al. described a retrospective analysis of 177 patients undergoing bronchoscopy with fluoroscopy, the diagnostic yield was found to be dependent on the location and size of the nodule (82% for central, 61% for intermediate and 53% for peripheral nodules), with particularly low yield for lesions <2 cm in the outer third of the lung (14%) (72). Aoshima et al. reported a diagnostic yield of 62% for malignant lesions and 12% for benign lesions, in a cohort of 208 bronchoscopy procedures carried out with fluoroscopy (73).
Oki et al. described a case series of 98 patients with peripheral pulmonary lesions undergoing fluoroscopic guided bronchoscopy with a 3.5 mm thin bronchoscope. The median lesion was 30.5 mm, and the overall diagnostic yield was 69% (74).
Guided bronchoscopic biopsy
Several guided bronchoscopy technologies have been developed to improve the yield of conventional bronchoscopy with TBB. These include navigational bronchoscopy such as electromagnetic navigational bronchoscopy (ENB), virtual bronchoscopy (VB), radial endobronchial ultrasound (rEBUS) with ultrathin bronchoscopy.
VB
This technology uses images from a chest CT scan to reconstruct a 3-dimensional map of the airways and the surrounding lung tissue. It is then used to create a bronchoscopic view and pathway from the trachea to the target lesion. A meta-analysis by Asano et al, showed a pooled diagnostic yield of 73%. The yield was lower (67%) for smaller lesions with a diameter <2 cm (75).
ENB
The addition of electromagnetic tracking to VB allows the bronchoscopists to use these virtual roadmaps to guide instruments to the SPN. A meta-analysis of the diagnostic yield of ENB showed a pooled diagnostic yield of 65% (76). A higher yield for ENB has been associated with the presence of bronchus sign leading to the SPN (77) (Figure 1), a lesion >3 cm (78), an upper lobe location (79), and the use of general anesthesia compared to conscious sedation (80).
In addition, some ENB system offers the additional flexibility of performing electromagnetic navigation with transthoracic needle aspiration (ETTNA) of the target lesion when the ENB bronchoscopic results are negative. In a study of 50 patients with varying SPN sizes, the overall diagnostic yield for such system was 83.3%. The yield was 77% for lesions without bronchus sign (81).
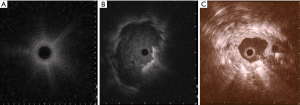
rEBUS
rEBUS offer real-time confirmation of the location of the SPN. The rEBUS image of normal lung parenchyma has a “snow- storm” appearance, whereas a solid lesion had a dark and “solid” appearance (Figure 5). The rEBUS can be used in combination with ENB or with an ultrathin bronchoscope. A recent systematic review of 57 studies and 7,872 lesions showed an overall diagnostic yield of 70.6%. The diagnostic yield was higher for lesions >2 cm in size, those associated with a bronchus sign and when the probe is located within the lesion as opposed to being adjacent to it (82,83) (Figure 5). A randomized study by Eberhardt et al. showed that ENB-assisted bronchoscopy combined with rEBUS is more sensitive than either modality alone (diagnostic yield of 88% vs. 69% and 59%, respectively) (79).
Ultrathin bronchoscope
This scope is much thinner than a standard flexible bronchoscope and has the ability to navigate beyond 5th or 6th order airways while retaining visualization. Ultrathin bronchoscopy is often combined with other techniques, such as VB or rEBUS for tissue sampling. Combination of rEBUS and ultrathin bronchoscopy has a reported overall diagnostic yield of 69% (84). The diagnostic yield for a lesion less than 2 cm was 36% compared with 77% for lesions larger than 2 cm.
Newer modalities
ENB biopsy is limited by the lack of real time confirmation of the location of the nodule. Intraprocedural cone-beam computed tomography (CBCT) imaging has been used to confirm the location of the lung nodule and overlay that location on live fluoroscopy imaging (augmented fluoroscopy). In a retrospective analysis of 75 patients who underwent ENB guided biopsy using intraoperative CBCT data with augmented fluoroscopy, a diagnostic yield of 83% was obtained. This yield was independent of the lesion size, location, fluoroscopic visibility or the presence of bronchus sign (85).
A robotic endoscopy system has been recently developed. It offers the potential of continuous direct visualization and precise control of the tools. In a small pilot feasibility study of 15 patients who have a suspected lesion with a bronchus sign, tissue acquisition under direct visualization was done in 14/15 (93%) patients. Cancer was confirmed in 9/15 (60%) patients, specific benign features were found in 5/15 (33%) patients and included necrotizing pneumonia, Loeffler syndrome, actinomycosis, surgical scar, and atypical mycobacteria. One patient had a non-diagnostic bronchoscopy and required surgical biopsy to confirm a malignant diagnosis. There were no reported adverse events (86).
Surgical resection
Surgical resection remains the gold standard diagnostic and therapeutic modality for suspicious pulmonary nodules. It is indicated in cases where the suspicion for malignancy remains high despite a negative/undetermined non-surgical work-up, or if the risk of malignancy is high enough to merit proceeding directly to resection. The decision for resection has to balance the benefits of a definite diagnosis/therapy with the surgical risk.
Surgical techniques include video-assisted thoracic surgery (VATS), open thoracotomy and robotic assisted thoracoscopic surgery (RATS). Despite the lack of studies directly comparing VATS with open approach, the former is preferred, due to its less invasive and morbid nature (3,6). In a propensity matched analysis using a Medicare database, 1,195 patients who underwent VATS were compared to 1,195 patients who underwent open lobectomy. The VATS group had a significantly lower rate of morbidities including atelectasis, postoperative pneumonia and sepsis. The hospital mortality was lower for VATS compared to open thoracotomy (2.1% vs. 3.6%; P=0.029) but the overall 3-year survival and disease-free survival were similar (87). In a retrospective study comparing RATS, VATS and open surgery for early stage lung cancer, median length of stay was shorter in the RATS compared to VATS and open surgery (4.5 and 6 days respectively; P<0.001) (63). Other propensity matched analysis showed that RATS and VATS had a similar postoperative morbidity and length of stay, which were significantly lower than open thoracotomy (88,89). No significant difference in long-term survival has been found among the three groups (89).
The initial approach is to perform a wedge resection whenever possible (which may be difficult for central nodules) with intraoperative frozen section pathology. If malignancy is seen on the frozen section, more extensive resection should be attempted. The extent of the final resection (wedge resection, segmentectomy, and lobectomy) depends on the location of the nodule and the presence of comorbidities. In patients who can tolerate a lobectomy, the procedure is recommended over a sublobar resection (segmentectomy/wedge). This recommendation is primarily derived from data in early-stage lung cancer, where lobectomy was associated with a trend toward survival benefit and a decrease in the rate of recurrence, primarily locoregional recurrence (90,91).
Stereotactic body radiotherapy (SBRT)
SBRT is currently the recommended therapy for stage I non-small cell lung cancer (NSCLC) who are not surgical candidate because of their comorbidities or for those who refuse surgery (2). In inoperable patients with early stage NSCLC, the 5-year local control rate of SBRT is reported to be above 90% (3,4). However, the efficacy of SBRT in operable patients remains unknown. Multiple randomized control trials in stage I NSCLC were closed prematurely due to low recruitment rate (5,6). A pooled analysis of 58 patients from these trials resulted in an estimated three year overall survival of 95% in the SBRT group compared to 79% in the surgery group (P=0.037) but the study has significant limitations (7).
Propensity score matching of retrospective data in patients with stage I–II NSCLC treated with VATS or SBRT shows mixed results. While some of these studies does not show any significant difference in the 3-year overall survival, disease free survival and freedom from local recurrence (8,9), other showed a significant advantage of VATS lobectomy compared to SBRT, with an overall five year survival of 68% vs. 37%, and recurrence free survival of 60% and 19% respectively (10).
SBRT target a small lung volume and has a low toxicity profile (3). Reported complications include grade 1 or 2 pneumonitis in 33–52% of the patients and grade 3 pneumonitis in 1% to 6%. Other minor complications such as rib pain, rib fracture, pleural effusion, hemoptysis and bacterial pneumonia have also been reported (8,9).
Functional preoperative evaluation
It is important to discuss the risks and benefits of the different therapeutic options, including surgical and non-surgical ones. Patients with low perioperative risk and high pretest probability for lung cancer may elect to proceed directly to surgery without tissue biopsy. On the other hand, patients with high perioperative risk need to be thoroughly evaluated to minimize perioperative morbidities, mortality and long-term disability.
The parameters to consider include age, the extent of planned resection, cardiac function, spirometry, diffusion capacity for carbon monoxide (DLCO), and the exercise capacity.
Post-operative mortality increases with age and with the extent of resection (92). Studies have shown a higher rate of lobectomy and sub-lobar resections in the elderly compared to younger patients with similar long term outcome (92,93). Age by itself in the absence of comorbidities does not constitute a contraindication to resection (94). The ACCP recommend to fully evaluate the functional status of all patients who are potential candidates for surgical resection regardless of their age (95).
The prevalence of coronary artery disease is high (11–17%) in patients with lung cancer. Cardiac consultation is recommended for patients with history of cardiac disease requiring medications, newly suspected cardiac disease, inability to climb 2 flights of stairs or a thoracic revised cardiac index (ThRCRI) ≥2 (96).
A lower absolute and percent predicted FEV1 has been associated with higher mortality. The BTS compiled data from more than 200 patients undergoing pulmonary resection. Using an absolute cut off value for FEV1 of >2 L for pneumonectomy and >1.5 L for lobectomy, the mortality rate was less than 5% (94). However, relying on absolute FEV1 creates a bias for older patients, women, and patients with shorter status and does not consider the functional contribution of the removed tissue.
The predicted post-operative FEV1 was shown to be a strong predictor of mortality and special attention to post-operative management is needed in patients with predicted post-operative FEV1 (ppoFEV1) of less than 30% (97). For a pneumonectomy, ppoFEV1 can be estimated using a perfusion scan by the following formula: ppoFEV1 = preoperative FEV1 × (1-fraction of total perfusion for the resected lung). For lobectomies, ppoFEV1 can be estimated using the following formula: ppoFEV1 = pre-operative FEV1 × (1 − a/b) with a being the number of unobstructed segments to be resected and b the total number of unobstructed segments (98).
DLCO has been found to correlate better with post-operative death than FEV1. A DLCO <60% of predicted was found to be associated with increased mortality (99,100).
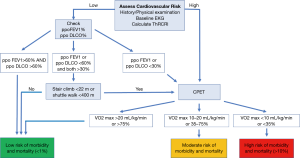
The ACCP recommends that further testing need to be done if the ppoFEV1 or ppoDLCO are expected to be less than 60% (95). If the patient is able to climb 5 flights of stairs (22 meters) or walk more than 400 meters on a shuttle walk test, the estimated operative mortality is low (1%) and he/she should be able to proceed to surgery (101). When the patient is unable to meet the above criteria, a symptom limited cardiopulmonary exercise test is recommended as it can assist in estimating the operative risk (95) (Figure 6).
Conclusions
SPN is a common finding in clinical practice. Determining the pretest probability of cancer should be the first step in the evaluation. This can be done by looking at specific risk factors such as age, smoking, location, size, type of the nodule (such as subsolid nodules), as well as the rate of progression when previous imaging are available. Validated models for risk stratification are available but clinical estimation may be as good. Further management will depend on the size and the type of the pulmonary nodule. Patient should be well informed of each approach’s risks and benefits and should be able to make an informed decision about potential diagnostic and therapeutic modalities.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society. AJR Am J Roentgenol 1984;143:509-17. [Crossref] [PubMed]
- Gurney JW. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part I. Theory. Radiology 1993;186:405-13. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Holin SM, Dwork RE, Glaser S, et al. Solitary pulmonary nodules found in a community-wide chest roentgenographic survey; a five-year follow-up study. Am Rev Tuberc 1959;79:427-39. [PubMed]
- Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. [Crossref] [PubMed]
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70 Suppl 2:ii1-ii54. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of Initial Low-Dose Computed Tomographic Screening for Lung Cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907-16. [Crossref] [PubMed]
- Veronesi G, Bellomi M, Spaggiari L, et al. Low dose spiral computed tomography for early diagnosis of lung cancer. Results of baseline screening in 5,000 high-risk volunteers. J Clin Oncol 2006;24:7029.
- Henschke CI, Yankelevitz DF, Naidich DP, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology 2004;231:164-8. [Crossref] [PubMed]
- Gohagan J, Marcus P, Fagerstrom R, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest 2004;126:114-21. [Crossref] [PubMed]
- Li F, Sone S, Abe H, et al. Malignant versus benign nodules at CT screening for lung cancer: comparison of thin-section CT findings. Radiology 2004;233:793-8. [Crossref] [PubMed]
- Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology 2003;226:756-61. [Crossref] [PubMed]
- Nawa T, Nakagawa T, Kusano S, et al. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest 2002;122:15-20. [Crossref] [PubMed]
- Diederich S, Wormanns D, Lenzen H, et al. Screening for asymptomatic early bronchogenic carcinoma with low dose CT of the chest. Cancer 2000;89:2483-4. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 2: pretest probability and algorithm. Chest 2013;143:840-6. [Crossref] [PubMed]
- Gould MK, Ananth L, Barnett PG. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007;131:383-8. [Crossref] [PubMed]
- Peto R, Lopez AD, Boreham J, et al. Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet 1992;339:1268-78. [Crossref] [PubMed]
- Quint LE, Park CH, Iannettoni MD. Solitary pulmonary nodules in patients with extrapulmonary neoplasms. Radiology 2000;217:257-61. [Crossref] [PubMed]
- Mery CM, Pappas AN, Bueno R, et al. Relationship between a history of antecedent cancer and the probability of malignancy for a solitary pulmonary nodule. Chest 2004;125:2175-81. [Crossref] [PubMed]
- Naccache JM, Gibiot Q, Monnet I, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 2018;10:3829-44. [Crossref] [PubMed]
- Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008;178:738-44. [Crossref] [PubMed]
- Fox DL, Muller NL. Pulmonary cryptococcosis in immunocompetent patients: CT findings in 12 patients. AJR Am J Roentgenol 2005;185:622-6. [Crossref] [PubMed]
- Murayama S, Sakai S, Soeda H, et al. Pulmonary cryptococcosis in immunocompetent patients: HRCT characteristics. Clin Imaging 2004;28:191-5. [Crossref] [PubMed]
- Primack SL, Muller NL. Radiologic manifestations of the systemic autoimmune diseases. Clin Chest Med 1998;19:573-86. vii. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94s-107s.
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332-41. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung adenocarcinoma in resected specimens: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013;137:685-705. [Crossref] [PubMed]
- Kim HY, Shim YM, Lee KS, et al. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 2007;245:267-75. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. [Crossref] [PubMed]
- Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849-55. [Crossref] [PubMed]
- Swensen SJ, Viggiano RW, Midthun DE, et al. Lung nodule enhancement at CT: multicenter study. Radiology 2000;214:73-80. [Crossref] [PubMed]
- Harders SW, Madsen HH, Rasmussen TR, et al. High resolution spiral CT for determining the malignant potential of solitary pulmonary nodules: refining and testing the test. Acta Radiol 2011;52:401-9. [Crossref] [PubMed]
- Winer-Muram HT. The solitary pulmonary nodule. Radiology 2006;239:34-49. [Crossref] [PubMed]
- Muram TM, Aisen A. Fatty metastatic lesions in 2 patients with renal clear-cell carcinoma. J Comput Assist Tomogr 2003;27:869-70. [Crossref] [PubMed]
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol 1980;135:1269-71. [Crossref] [PubMed]
- Woodring JH, Fried AM. Significance of wall thickness in solitary cavities of the lung: a follow-up study. AJR Am J Roentgenol 1983;140:473-4. [Crossref] [PubMed]
- Nietert PJ, Ravenel JG, Leue WM, et al. Imprecision in automated volume measurements of pulmonary nodules and its effect on the level of uncertainty in volume doubling time estimation. Chest 2009;135:1580-7. [Crossref] [PubMed]
- Gaeta M, Caruso R, Blandino A, et al. Radiolucencies and cavitation in bronchioloalveolar carcinoma: CT-pathologic correlation. Eur Radiol 1999;9:55-9. [Crossref] [PubMed]
- Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177-84. [Crossref] [PubMed]
- Soubani AO. The evaluation and management of the solitary pulmonary nodule. Postgrad Med J 2008;84:459-66. [Crossref] [PubMed]
- Revel MP, Merlin A, Peyrard S, et al. Software volumetric evaluation of doubling times for differentiating benign versus malignant pulmonary nodules. AJR Am J Roentgenol 2006;187:135-42. [Crossref] [PubMed]
- Larici AR, Farchione A, Franchi P, et al. Lung nodules: size still matters. Eur Respir Rev 2017;26:170025. [Crossref] [PubMed]
- Wang CW, Teng YH, Huang CC, et al. Intrapulmonary lymph nodes: computed tomography findings with histopathologic correlations. Clin Imaging 2013;37:487-92. [Crossref] [PubMed]
- Nolop KB, Rhodes CG, Brudin LH, et al. Glucose utilization in vivo by human pulmonary neoplasms. Cancer 1987;60:2682-9. [Crossref] [PubMed]
- Shaham D, Vazquez M, Bogot NR, et al. CT features of intrapulmonary lymph nodes confirmed by cytology. Clin Imaging 2010;34:185-90. [Crossref] [PubMed]
- de Hoop B, van Ginneken B, Gietema H, et al. Pulmonary perifissural nodules on CT scans: rapid growth is not a predictor of malignancy. Radiology 2012;265:611-6. [Crossref] [PubMed]
- Mets OM, Chung K, Scholten ET, et al. Incidental perifissural nodules on routine chest computed tomography: lung cancer or not? Eur Radiol 2018;28:1095-101. [Crossref] [PubMed]
- Chang CY, Tzao C, Lee SC, et al. Incremental value of integrated FDG-PET/CT in evaluating indeterminate solitary pulmonary nodule for malignancy. Mol Imaging Biol 2010;12:204-9. [Crossref] [PubMed]
- Yi CA, Lee KS, Kim BT, et al. Tissue characterization of solitary pulmonary nodule: comparative study between helical dynamic CT and integrated PET/CT. J Nucl Med 2006;47:443-50. [PubMed]
- Kim SK, Allen-Auerbach M, Goldin J, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med 2007;48:214-20. [PubMed]
- Veronesi G, Travaini LL, Maisonneuve P, et al. Positron emission tomography in the diagnostic work-up of screening-detected lung nodules. Eur Respir J 2015;45:501-10. [Crossref] [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005;128:2490-6. [Crossref] [PubMed]
- Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest 2014;145:464-72. [Crossref] [PubMed]
- Balekian AA, Silvestri GA, Simkovich SM, et al. Accuracy of clinicians and models for estimating the probability that a pulmonary nodule is malignant. Ann Am Thorac Soc 2013;10:629-35. [Crossref] [PubMed]
- Swensen SJ, Silverstein MD, Edell ES, et al. Solitary pulmonary nodules: clinical prediction model versus physicians. Mayo Clin Proc 1999;74:319-29. [Crossref] [PubMed]
- Tanner NT, Porter A, Gould MK, et al. Physician Assessment of Pretest Probability of Malignancy and Adherence With Guidelines for Pulmonary Nodule Evaluation. Chest 2017;152:263-70. [Crossref] [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-e65S.
- Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. [Crossref] [PubMed]
- Kakinuma R, Muramatsu Y, Kusumoto M, et al. Solitary Pure Ground-Glass Nodules 5 mm or Smaller: Frequency of Growth. Radiology 2015;276:873-82. [Crossref] [PubMed]
- Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules >/= 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Yankelevitz DF, Yip R, Smith JP, et al. CT Screening for Lung Cancer: Nonsolid Nodules in Baseline and Annual Repeat Rounds. Radiology 2015;277:555-64. [Crossref] [PubMed]
- Cohen JG, Reymond E, Lederlin M, et al. Differentiating pre- and minimally invasive from invasive adenocarcinoma using CT-features in persistent pulmonary part-solid nodules in Caucasian patients. Eur J Radiol 2015;84:738-44. [Crossref] [PubMed]
- Sánchez M, Benegas M, Vollmer I. Management of incidental lung nodules <8 mm in diameter. J Thorac Dis 2018;10:S2611-27. [Crossref] [PubMed]
- Soardi GA, Perandini S, Motton M, et al. Assessing probability of malignancy in solid solitary pulmonary nodules with a new Bayesian calculator: improving diagnostic accuracy by means of expanded and updated features. Eur Radiol 2015;25:155-62. [Crossref] [PubMed]
- Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. [Crossref] [PubMed]
- Ost DE, Ernst A, Lei X, et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med 2016;193:68-77. [Crossref] [PubMed]
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049-54. [Crossref] [PubMed]
- Aoshima M, Chonabayashi N. Can HRCT Contribute in Decision-Making on Indication for Flexible Bronchoscopy for Solitary Pulmonary Nodules and Masses? J Bronchology Interv Pulmonol 2001;8:161-5.
- Oki M, Saka H, Kitagawa C, et al. Novel thin bronchoscope with a 1.7-mm working channel for peripheral pulmonary lesions. Eur Respir J 2008;32:465-71. [Crossref] [PubMed]
- Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration 2014;88:430-40. [Crossref] [PubMed]
- Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration 2014;87:165-76. [Crossref] [PubMed]
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a Bronchus sign on CT imaging: results from a prospective study. Chest 2010;138:1316-21. [Crossref] [PubMed]
- Gildea TR, Mazzone PJ, Karnak D, et al. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med 2006;174:982-9. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36-41. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Raval AA, Amir L. Community hospital experience using electromagnetic navigation bronchoscopy system integrating tidal volume computed tomography mapping. Lung Cancer Manag 2016;5:9-19. [Crossref] [PubMed]
- Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017;22:443-53. [Crossref] [PubMed]
- Chen A, Chenna P, Loiselle A, et al. Radial probe endobronchial ultrasound for peripheral pulmonary lesions. A 5-year institutional experience. Ann Am Thorac Soc 2014;11:578-82. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Endobronchial ultrasound-guided transbronchial biopsy using novel thin bronchoscope for diagnosis of peripheral pulmonary lesions. J Thorac Oncol 2009;4:1274-7. [Crossref] [PubMed]
- Pritchett MA, Schampaert S, de Groot JAH, et al. Cone-Beam CT With Augmented Fluoroscopy Combined With Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J Bronchology Interv Pulmonol 2018;25:274-82. [Crossref] [PubMed]
- Rojas-Solano JR, Ugalde-Gamboa L, Machuzak M. Robotic Bronchoscopy for Diagnosis of Suspected Lung Cancer: A Feasibility Study. J Bronchology Interv Pulmonol 2018;25:168-75. [PubMed]
- Paul S, Isaacs AJ, Treasure T, et al. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ 2014;349:g5575. [Crossref] [PubMed]
- Kneuertz PJ, Singer E, D'Souza DM, et al. Hospital cost and clinical effectiveness of robotic-assisted versus video-assisted thoracoscopic and open lobectomy: A propensity score-weighted comparison. J Thorac Cardiovasc Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Detterbeck FC. Lobectomy versus limited resection in T1N0 lung cancer. Ann Thorac Surg 2013;96:742-4. [Crossref] [PubMed]
- Damhuis RA, Schutte P. Resection rates and postoperative mortality in 7,899 patients with lung cancer. Eur Respir J 1996;9:7-10. [Crossref] [PubMed]
- de Perrot M, Licker M, Reymond M, et al. Influence of age on operative mortality and long-term survival after lung resection for bronchogenic carcinoma. Eur Respir J 1999;14:419-22. [Crossref] [PubMed]
- British Thoracic Society. Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-e90S.
- Brunelli A, Varela G, Salati M, et al. Recalibration of the revised cardiac risk index in lung resection candidates. Ann Thorac Surg 2010;90:199-203. [Crossref] [PubMed]
- Nakahara K, Ohno K, Hashimoto J, et al. Prediction of postoperative respiratory failure in patients undergoing lung resection for lung cancer. Ann Thorac Surg 1988;46:549-52. [Crossref] [PubMed]
- Bolliger CT, Gückel C, Engel H, et al. Prediction of Functional Reserves after Lung Resection: Comparison between Quantitative Computed Tomography, Scintigraphy, and Anatomy. Respiration 2002;69:482-9. [Crossref] [PubMed]
- Ferguson MK, Little L, Rizzo L, et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg 1988;96:894-900. [PubMed]
- Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg 2008;85:1158-64; discussion 1164-5. [Crossref] [PubMed]
- Brunelli A, Refai M, Xiume F, et al. Performance at symptom-limited stair-climbing test is associated with increased cardiopulmonary complications, mortality, and costs after major lung resection. Ann Thorac Surg 2008;86:240-7; discussion 247-8. [Crossref] [PubMed]