
Fine-tuning autophagy in pancreatic adenocarcinoma: full blockage is required
Autophagy is a physiological process of cellular component recycling promoting cell survival and homeostasis under unfavorable conditions. Pancreatic ductal adenocarcinoma (PDAC) (90–95% of pancreatic cancer) has been associated with multiple metabolic alterations including autophagy. Because of its supporting role in tumor growth, inhibition of autophagy has been proposed as a treatment of pancreatic cancer (1,2). Autophagy process is regulated by autophagy-related proteins (ATGs) necessary for the autophagosome formation and subsequent delivering of cytoplasmic content for degradation by lysosomes. Among these proteins, Atg5 is a key player for autophagy onset but little is known about its role in tumorigenesis (3).
Ryan’s laboratory previously deciphered the roles of Atg5/7 in pancreatic carcinogenesis and notably showed the importance of p53 status (4). They observed that mice lacking Atg5 accumulate low-grade pancreatic intraepithelial neoplasia (PanIN) lesions and that PDAC progression is impaired. Bardeesy’s laboratory demonstrated the induction of an autophagy-lysosome gene program regulating metabolic reprogramming in pancreatic adenocarcinoma highlighting the potential of therapeutic lysosome targeting using chloroquine (CQ) (5).
In the later elegant study, Görgülü and colleagues investigated the dosage sensitive effect of autophagy loss (6). Using GFP-LC3 reporter mice, autophagy was confirmed to occur at every step from acinar-to-ductal metaplasias (ADM), PanINs, pancreatic tumors to metastasis. The authors crossed the prototype mice model expressing oncogenic mutated LstopL-KrasG12D in pancreatic cells (deriving from exocrine Ptf1 cell lineage) with mice carrying conditional disruption of Atg5 (Atg5flox/flox) and compared homozygous (called A5;Kras) and heterozygous (called A5+/−;Kras) with mice harbouring mutated KrasG12D (littermate control). Isolated primary tumor cells were characterized regarding their transcriptome or metabolome and more targeting features such as intracellular calcium, activity of extracellular cathepsin, and cell migration/invasion.
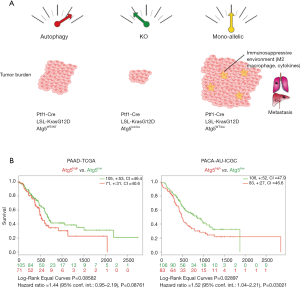
As expected, homozygous A5/Kras did not develop any advanced tumor. This was consistent with previous observations (4). Strikingly, monoallelic Atg5 loss (A5+/–;Kras mice), expressing haploinsufficient Atg5 levels, did not have an intermediate phenotype but harboured an increased burden of tumors and metastases than control mice (Figure 1A). In vitro culture of A5+/−;Kras cells showed that monoallelic Atg5 loss led to anoikis resistance, increased migration, invasion and cell spreading. Similar observations were independently obtained using Atg5-ShRNA Kras cell lines harbouring 58% or 94% Atg5 relative levels (mimicking A5+/−;Kras and A5;Kras cells, respectively).
Transcriptomic analysis showed that monoallelic Atg5 loss induced a transcriptional reprogramming with increased expression of genes involved in metabolism, immunity, development, and vesicular trafficking/homeostasis and decreased of adhesion and cell cycle associated cellular functions.
The A5+/−;Kras pancreas displayed a profound metabolic reprogramming. Indeed, the authors observed elevated amount of oxidative and cell stress associated metabolites. The mitochondrial function and morphology were altered with global decrease of glycolysis, oxidative capacity, acidification rate and ATP turnover in A5+/− cells compared with Kras controls. Cytoplasmic Ca2+ amplitude response and s100a4 (Ca2+ binding protein) were increased in A5+/− cells as well as L- and D-cathepsin activities required for spreading and invasive capacities.
Görgülü and colleagues also investigated the consequence of mono-allelic Atg5 loss on macrophage-mediated inflammation. Cytokine pattern of A5+/− cells was altered with the up-regulation of sets of cytokines involved in macrophage chemoattraction, M2-differentiation and tumor progression/metastasis and decrease of M1 related cytokines. Therefore, A5+/−;Kras cells switched the differentiation pattern from M1 macrophage toward the M2-subtype. M2 macrophages are tumor-associated macrophages (TAM) that contribute to immunosuppressive microenvironment and favor tumor aggressiveness and promote tumor metastasis (7).
Finally, analysis of three independent patient’s cohorts (from Munich, Heidelberg and Berlin, Germany) showed a tendency of poorer survival in the group expressing moderate/low ATG5 level compared to high expressing group. Using SurvExpress tool (8), we observed an opposite trend when comparing high and low expressing Atg5 patients from PAAD-TCGA (P=0.08; n=176) and PACA-AU-ICGC (P=0.03; n=189) datasets (Figure 1B). In this kind of analysis, it would be interesting to stratify patient with low and moderate autophagy features.
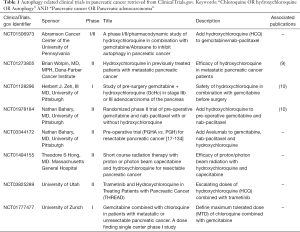
Altogether, this interesting manuscript highlights the critical need of a complete blockage of the autophagy for clinical trials targeting autophagy. CQ is approved by the US Food and Drug Administration, FDA and widely employed for the prophylactic malaria medication. The therapeutic potential of CQ/nivaquine has been tested for cancers. Several studies have been conducted for pancreatic adenocarcinoma (Table 1). In regards of the recent findings, the residual activity of autophagy should be evaluated in order to avoid the induction of protumoral immunosuppressive environment described by Algül’s laboratory (6).
Full table
Acknowledgements
Funding: Our work is supported by Inserm and CNRS and grants from la Ligue Nationale Contre le Cancer (Equipe Labellisée Ligue 2010, IVS; Ligue comité 59, 62 IVS, Ligue comité 62, 80 NJ) and by SIRIC ONCOLille, Grant INCaDGOS-Inserm 6041 (NJ, IVS) and by a grant from “Contrat de Plan Etat Région” CPER Cancer 2007-2013 (IVS).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yang A, Herter-Sprie G, Zhang H, et al. Autophagy Sustains Pancreatic Cancer Growth through Both Cell-Autonomous and Nonautonomous Mechanisms. Cancer Discov 2018;8:276-87. [Crossref] [PubMed]
- Boone BA, Zeh HJ 3rd, Bahary N. Autophagy Inhibition in Pancreatic Adenocarcinoma. Clin Colorectal Cancer 2018;17:25-31. [Crossref] [PubMed]
- Liu H, He Z, Simon HU. Protective role of autophagy and autophagy-related protein 5 in early tumorigenesis. J Mol Med (Berl) 2015;93:159-64. [Crossref] [PubMed]
- Rosenfeldt MT, O'Prey J, Morton JP, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature 2013;504:296-300. [Crossref] [PubMed]
- Perera RM, Stoykova S, Nicolay BN, et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature 2015;524:361-5. [Crossref] [PubMed]
- Görgülü K, Diakopoulos KN, Ai J, et al. Levels of the Autophagy-Related 5 Protein Affect Progression and Metastasis of Pancreatic Tumors in Mice. Gastroenterology 2019;156:203-217.e20. [Crossref] [PubMed]
- Najafi M, Goradel NH, Farhood B, et al. Tumor microenvironment: Interactions and therapy. J Cell Physiol 2019;234:5700-21. [Crossref] [PubMed]
- Aguirre-Gamboa R, Gomez-Rueda H, Martinez-Ledesma E, et al. SurvExpress: an online biomarker validation tool and database for cancer gene expression data using survival analysis. PLoS One 2013;8:e74250. [Crossref] [PubMed]
- Wolpin BM, Rubinson DA, Wang X, et al. Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. Oncologist 2014;19:637-8. [Crossref] [PubMed]
- Boone BA, Murthy P, Miller-Ocuin J, et al. Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps. BMC Cancer 2018;18:678. [Crossref] [PubMed]


