
Nephron repair: powered by anaerobic energy metabolism
Acute kidney injury (AKI) is clinically classified as abrupt organ damage that occurs within a short time period spanning a few hours or days (1). AKI commonly attacks anatomical structures called nephrons, which are tubular units responsible for conducting the essential excretory and osmoregulatory functions of the kidney. AKI frequently has fatal consequences, but this condition is highly heterogeneous and patients can exhibit partial or complete restoration of renal activities (1). There have been many critical advances in understanding the molecular basis of kidney damage and subsequent tissue regeneration in recent years (2). Despite such progress, new treatments that definitively improve outcomes for AKI patients have not been identified, and thus there remains an urgent necessity to discover useful therapeutic approaches (3). A challenge in this regard is that many aspects of renal injury and subsequent reparative and/or regenerative processes remain opaque, while others are highly controversial due to conflicting studies, such as the involvement of endogenous stem cells (2). Therefore, it is pivotal to uncover further insights into the cellular and genetic mechanisms that regulate the response to kidney injury.
Several aspects of nephron injury and the reaction to damage have been difficult or wholly intractable to investigate due to the limitations posed by current experimental models, which to date have predominantly relied on the use of rodent species (4). For example, some knowledge gaps have persisted due to the inaccessibility of in vivo monitoring of the renal injury and subsequent cellular responses in these mammalian models. Fortunately, the zebrafish system has surfaced as a new and valuable opportunity for direct visualization of such biological activities due to their optical transparency, small size, and rapid ex utero development (5). At just 48 hours post fertilization (hpf), larval zebrafish have formed a functional embryonic kidney, known as the pronephros, which filters the circulation to perform excretion and maintain fluid homeostasis (6). The zebrafish pronephros provides a simplified platform to visualize cellular dynamics because it consists of just two parallel nephron units. The size and transparency of the embryo over the first week of development enables direct observation of the nephron cells and their physiological activities non-invasively within the living organism using readily available confocal microscopy and time lapse imaging technology (6). By comparison, the rodent kidney is located deep within the body, and has several thousand nephrons, making it impossible to monitor nephron dynamics. Further, despite the formulation of protocols to explant the mouse renal primordium forin vitro organ culture, using this method to examine the consequences of nephron injury is not feasible due to the limits of tissue survival time with current techniques (7).
Importantly, the zebrafish pronephros is comprised of evolutionarily conserved structures with analogous functions. Similar to other vertebrates, including humans, zebrafish nephrons consist of a blood filter, segmented tubule, and collecting duct (8,9). Within these compartments, cells of the zebrafish exhibit histological and molecular similarities as well, including the segment-specific expression of solute transporter proteins and development of primary cilia, though there are some distinctions like the presence of multiciliated cells (8-10). The pronephros arises from bilateral stripes of renal progenitors that undergo a mesenchymal to epithelial transition by 24 hpf (11,12). While these nephrons are initially linear, they undergo progressive coiling morphogenesis between 26 and 144 hpf that transforms the straight nephron into a structure with a characteristic convolution of the proximal tubule as typical of most other vertebrates (8). This morphogenesis is driven first by a posterior-to-anterior collective cell migration of tubule cells, and later supported by proliferation in the distal tubule segments (13). Thus, insights into processes ranging from nephron cell specification to differentiation, physiology and behavior can be achieved by studying the zebrafish pronephros.
Interestingly, it has been discovered that the zebrafish pronephros tubule can recover following some, but not all, acute injuries. For example, after laser-induced cell ablation of a small stretch of tubule epithelial cells, there is rapid restoration of the gap to form a continuous layer of epithelium (14,15). This repair is fueled initially by collective cell migration of neighboring epithelial cells, and subsequently by proliferation at more distant sites where cell stretching accommodates the migratory movement (15). This phenomenon closely mirrors the normal developmental events that facilitate proximal segment convolution (13). However, exposure to the antibiotic gentamicin, which induces widespread proximal tubule injury, is lethal to the embryo and thus has been a challenging context in which to study epithelial regeneration (16). In spite of this limitation, the other attributes of the zebrafish model can be leveraged to facilitate the identification of novel renal regenerative mechanisms that can be interrogated further in mammals.
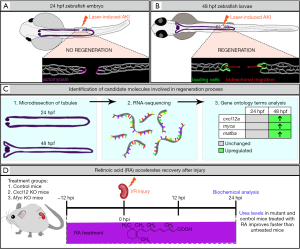
In a recent issue of Nature Communications, an exciting report authored by Yakulov et al. (17), demonstrates how the zebrafish pronephros can be implemented as a springboard for the discovery of conserved factors that promote kidney regeneration after acute injury using the aforementioned laser injury technique. The investigators used a 2-photon laser to perform localized cell ablations to elicit focal AKI in cldn2b:lyn-GFP transgenic embryos where plasma membranes of pronephros cells were labeled by green fluorescent protein (GFP), enabling them to track the tubule response to insult (Figure 1A,B). Upon injuring different ages of zebrafish, the authors noticed that animals with damage inflicted after 36 hpf exhibited successful repair, where both ends of the injured tubule migrated toward one another to close the gap induced by ablation (Figure 1B). However, if the laser damage was inflicted before 30 hpf, the embryos failed to undergo tubule reconstruction (Figure 1A). Unexpectedly, it was found that these differently aged animals displayed divergent phenotypes in the immediate response to injury. The authors observed that embryos aged 30 hpf or younger subjected to nephron ablation underwent the accumulation and contraction of actomyosin bundles in cells situated adjacent to the injury site, leading to the occlusion of each respective side (Figure 1A). In these cases, the connection between the occluded tubules was not restored over time, leaving a permanent gap. Conversely, embryos that experienced nephron ablation at ages >36 hpf displayed no apparent upregulation of actomyosin. Rather, they exhibited a bidirectional migratory response of the surviving tubule cells, which transiently supersedes the posterior-to-anterior cell migration program that typifies nephron morphogenesis (Figure 1B). The result was the restoration of a continuous tubule structure.
These studies are the first to document a developmental switch between repair mechanisms during nephron maturation. It is puzzling that late, but not early, injury to the pronephros was repairable. Indeed, high cellular plasticity is typically associated with early embryogenesis and provides a bastion against irreversible damage in many developing tissues and organs. The benefits of an occlusion response to tubule injury are mysterious, though as speculated by the authors, could very possibly be to stave off disturbances to water or electrolyte homeostasis at early stages in embryogenesis (17).
Next, the investigators uncovered surprising new insights into the role of fluid flow in the response to nephron injury. It has been proposed that fluid flow originating at the glomerular blood filter, and then propagated by the beating action of tubular cilia, is required to support collective cell migration. This proposal is based on the result of mechanical obstruction to the pronephros, which was found to abolish morphogenesis of the proximal tubule and block migration of this populace (13). Remarkably, when Yakulov et al. (17) performed ablation injuries at 48 hpf on flanking sides of the occlusions resultant from a primary ablation that had been introduced before 30 hpf, the investigators found that each side was independently restored by collective cell migration in spite of the permanent intervening gap between the two sides. This is an unforeseen result because the integrity of fluid flow within the tubule was interrupted by the primary injury, thus abrogating the connection for fluid to enter the downstream tubule section. These data indicate that injury-induced migration occurs independently of glomerular fluid flow. In the separated downstream tubule, it is likely that some fluid transport into the pronephric lumen occurs by active solute transport across the epithelium, which could be propagated by ciliated cells to create an independent source of fluid flow. However, Yakulov et al. (17) also tested the response to nephron injury in Ift88 mutants that have resultant ciliary defects, and found that the disruption of ciliogenesis did not affect the migratory repair response. This is particularly fascinating given that cilium mechanotransduction to sense fluid flow has been implicated in the response to kidney disease and acute damage (18). Taken together, these results disentangle fluid flow and ciliary function from the mechanisms that trigger nephron epithelial cells to undergo a migratory-based recovery.
Following their discovery of the developmental switch between these two different repair responses, the authors subsequently explored the possible mechanisms underlying the migratory repair response. In mammals, there is considerable evidence that nephron tubule damage is followed by the dedifferentiation of nearby epithelial cells to undergo a mesenchymal transition (EMT) that fuels restoration of the tubule (18,19). Hallmarks of this transition have been documented based on the expression of mesenchymal markers as well as stem/progenitor markers such as the Pax2 transcription factor (19). Further, when chemical induced injury is triggered using the antibiotic gentamicin, this causes high levels of proximal tubule damage in the zebrafish embryo and adult, in which surviving cells re-express the ortholog Pax2a, suggesting a dedifferentiation mechanism is occurring (20,21). Through a number of subsequent studies to assess gene expression, Yakulov et al. explored whether they could detect hallmarks of dedifferentiation during the response to laser injury in the pronephros. Interestingly, they did not identify alterations in the expression of transcripts encoding pax2a or other genes typically associated with EMT, such as vim1, snail1, netrin or mmp9. Further, the authors determined that the development of cell polarity did not impact the nephron migratory repair response, where neither disruption of canonical Wnt signaling, non-canonical Wnt signaling pathway, nor the apical-basal polarity complex member encoded by par6b was deleterious.
Next, the identification of these two distinct repair capacities served as the cornerstone for pinpointing molecules involved in tubule regeneration. In order to identify differentially expressed genes in these two conditions, RNA-sequencing was performed on micro-dissected 24 and 48 hpf pronephric tubules (Figure 1C). Gene expression profiles revealed candidate molecules cxcl12a, mafba and myca (Figure 1C), which were enriched in the older nephron tubule populace. Notably, CXCL12/CXCR4 signaling drives directional tissue migration during a multitude of biological processes ranging from organogenesis to tumor metastasis (22). Further, the mammalian orthogue of myca, Myc, can induce a polycystic kidney disease phenotype when overexpressed in the murine adult kidney and is upregulated in humans and mice with mutations in the PKD1 and PKD2 genes (23).
Thus, Yakulov et al. (17) selected these candidates for further studies, which included testing of the nephron tubule migratory repair response in cxcl12a-, cxcr4b-, and myca-deficient zebrafish embryos independently. After laser ablation, these various mutant zebrafish lacked an organized migratory response, which led to defective tubule repair. Additionally, these results in zebrafish paralleled phenotypes observed in mice with inducible, kidney specific deletion of Cxcl12 and Myc. Upon ischemia/reperfusion (IR) injury, mutant mice manifested higher serum urea levels and elevated urinary NGAL excretion as compared to control mice. Histological differences in renal tissues were not apparent, likely indicating that the delayed recovery had underlying physiological effects.
In order to distinguish downstream target pathways, Yakulov et al. (17) performed RNA-sequencing of injured Cxcl12 and Myc-deficient mouse kidneys. Gene-set enrichment analysis revealed that both knockout (KO) kidneys possessed downregulated metabolic and mitochondrial gene expression signatures. Interestingly, the investigators detected significant alterations in retinoic acid (RA) signaling. Because RA has been shown to improve mitochondrial function (24), the authors tested if this mitogen could rescue delayed renal recovery in Myc KO mice. Treatment with all-trans-RA (tretinoin) accelerated kidney recovery after I/R injury in wild-type and mutant mice (Figure 1D). To further probe the defective metabolic mechanisms delaying recovery in Cxcl12 and Myc KO mice, urinalysis after injury was performed. It was found that branched chain amino acid levels were drastically elevated in the mutant mice as compared to wild-type controls, indicating inoperative glycolysis and gluconeogenesis. This was further substantiated by in vitro cell culture studies where the investigators isolated tubular epithelial cells from wild-type controls and Myc KO mice. Here, upon examination of glycolytic capacity, the team found that mutant cells were impaired in eliciting a maximal glycolytic response.
Given the intractability of studying intranephron dynamics in vivo with their mammalian models, the investigators then returned to the implementation of the zebrafish nephron laser ablation model to visualize if and how changes in glycolysis affected cell migration after injury. Here, they cleverly took advantage of the amenability of zebrafish embryos for chemical genetics (5). Yakulov et al. (17) selected a phosphofructokinase-2/fructose-2,6,-bisphosphatase inhibitor, the small molecule 3-(3-pyri-dinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO), or the non-metabolizable glucose analog 2-deoxy-D-glucose (2DG), to transiently reduce glycolysis. Interestingly, when embryos with tubule ablations were subjected to liquid media containing 20 µM 3PO or 40 µM 2-DG, there was a transitory effect on the initial migratory reparative response leading to a reduced incidence of repaired nephron injuries at 6 hours post damage. Although interference with glycolysis using either compound slowed the epithelial migratory repair response, it did not prevent pronephros regeneration, as nearly all injuries were repaired 24 hours post damage. Similar effects of delayed migration were observed in the formation of lateral line as well. It is possible that these outcomes were impacted by transient effect and/or the stability of these drugs over time, where multiple applications could be used to further explore this point. Nevertheless, these results suggest CXCL12 and MYC regulate fast cell movements in migrating populations by operating glycolysis.
Overall, this study is a testament to the utility of complementary application of murine and zebrafish models to investigate the pathways involved in AKI. Perhaps most valuable is that their work has revealed for the first time that CXCL12 and MYC are necessary within the kidney for increasing cell glycolysis in the aftermath of renal cell damage. Manifold differences exist in the scope and nature of damage between I/R injury in a murine adult kidney compared to focal tubule epithelium ablation in the zebrafish pronephros. Nevertheless, it is fascinating that the response to these injuries invokes a metabolic switch in surviving cells that has potent effects in the aftermath of the sudden damage, where time lapse imaging in the zebrafish revealed that this glycolytic response facilitates rapid cellular migration that accelerates repair. Whether cell migration occurs in the mammalian context is not readily addressable with current methods, though the cell culture studies support the notion that there may be parallels in that nephron tubular cells require Myc for glycolytic capacity. Future studies examining mafba as a potential player in renal injury response are likely to be fruitful due to its localized mRNA expression pattern within the podocytes and proximal convoluted tubule during zebrafish pronephros development (8), and its previous history in driving a cellular migration process necessary for zebrafish lymphatic vessel development (25). Taken together, Yakulov et al. have contributed novel and exciting insights about how vertebrate kidney organs respond to injury through dynamic metabolic changes with their multisystem studies.
Acknowledgements
Funding: This work was supported in part by funds from the National Institutes of Health, Grant R01DK100237 to RAW, and a graduate fellowship from the University of Notre Dame to BEC.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care 2016;20:299. [Crossref] [PubMed]
- Kumar S. Cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int 2018;93:27-40. [Crossref] [PubMed]
- de Caestecker M, Harris R. Translating knowledge into therapy for acute kidney injury. Semin Nephrol 2018;38:88-97. [Crossref] [PubMed]
- Zuk A, Bonventre JV. Acute kidney injury. Annu Rev Med 2016;67:293-307. [Crossref] [PubMed]
- Poureetezadi SJ, Wingert RA. Little fish, big catch: zebrafish as a model for kidney disease. Kidney Int 2016;89:1204-10. [Crossref] [PubMed]
- Gerlach GF, Wingert RA. Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip Rev Dev Biol 2013;2:559-85. [Crossref] [PubMed]
- Rak-Raszewska A, Hauser PV, Vainio S. Organ in vitro culture: what have we learned about early kidney development? Stem Cells Int 2015;2015:959807. [Crossref] [PubMed]
- Wingert RA, Selleck R, Yu J, et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet 2007;3:1922-38. [Crossref] [PubMed]
- Wingert RA, Davidson AJ. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev Dyn 2011;240:2011-27. [Crossref] [PubMed]
- Marra AN, Li Y, Wingert RA. Antennas of organ morphogenesis: the roles of cilia in vertebrate kidney development. Genesis 2016;54:457-69. [Crossref] [PubMed]
- Gerlach GF, Wingert RA. Zebrafish pronephros tubulogenesis and epithelial identity maintenance are reliant on the polarity proteins Prkc iota and zeta. Dev Biol 2014;396:183-200. [Crossref] [PubMed]
- McKee R, Gerlach GF, Jou J, et al. Temporal and spatial expression of tight junction genes during zebrafish pronephros development. Gene Expr Patterns 2014;16:104-13. [Crossref] [PubMed]
- Vasilyev A, Liu Y, Mudumana S, et al. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol 2009;7:e9. [Crossref] [PubMed]
- Johnson CS, Holzemer NF, Wingert RA. Laser ablation of the zebrafish pronephros to study renal epithelial regeneration. J Vis Exp 2011;54:e2845. [PubMed]
- Palmyre A, Lee J, Ryklin G, et al. Collective epithelial migration drives kidney repair after acute injury. PLoS One 2014;9:e101304. [Crossref] [PubMed]
- McCampbell KK, Wingert RA. New tides: using zebrafish to study renal regeneration. Transl Res 2014;163:109-22. [Crossref] [PubMed]
- Yakulov TA, Todkar AP, Slanchev K, et al. CXCL12 and MYC Control Energy Metabolism to Support Adaptive Responses After Kidney Injury. Nat Commun 2018;9:3660. [Crossref] [PubMed]
- Deane JA, Ricardo SD. Emerging roles for renal primary cilia in epithelial repair. Int Rev Cell Mol Biol 2012;293:169-93. [Crossref] [PubMed]
- McCampbell KK, Wingert RA. Renal stem cells: fact or science fiction? Biochem J 2012;444:153-68. [Crossref] [PubMed]
- Cianciolo Cosentino C, Skrypnyk NI, et al. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol 2013;24:943-53. [Crossref] [PubMed]
- McCampbell KK, Springer KN, Wingert RA. Atlas of cellular dynamics during zebrafish adult kidney regeneration. Stem Cells Int 2015;2015:547636. [Crossref] [PubMed]
- Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells 2005;23:879-94. [Crossref] [PubMed]
- Trudel M. c-Myc signalling in the genetic mechanism of polycystic kidney disease. In: Li X. editor. Polycystic Kidney Disease. Brisbane: Codon Publications, 2015:231-57.
- Zhang R, Wang Y, Li R, et al. Transcriptional factors mediating retinoic acid signals in the control of energy metabolism. Int J Mol Sci 2015;16:14210-44. [Crossref] [PubMed]
- Koltowska K, Paterson S, Bower NI, et al. mafba is a downstream transcriptional effector of Vegfc signaling essential for embryonic lymphangiogenesis in zebrafish. Genes Dev 2015;29:1618-30. [Crossref] [PubMed]


