Placebo-driven clinical trials of yeast-derived β-(1,3) glucan in children with chronic respiratory problems
Introduction
Forty years ago, β-glucans were first described as biological response modifiers (BRM) that could stimulate tumor rejection in mice. As with many other BRM, they were classified as “non-specific” because their molecular target(s) were unknown and their effects appeared to be pleiotropic and unpredictable. Nevertheless, there is extensive literature regarding the activity of β-glucans in animal tumor models (1) and, for the past 30 years, Japan has used several forms of mushroom-derived β-glucan in cancer patients (2).
Our research has shown that CR3 serves as a major receptor for β-glucans with human or mouse leukocytes and is probably responsible for all reported functions of β-glucans. Unlike other non-specific BRM, β-glucan specifically targets macrophages, neutrophils, and NK cells to tumors that are opsonized with antibodies and C3 (3). Therefore, β-glucan has the same specificity as the tumor-opsonizing antibodies. This research, in particular, has shown the therapeutic value in mice of small β-glucans that bind to CR3 and prime the receptor for subsequent cytotoxic activation if, and only if, membrane CR3 is subsequently clustered by contact with the clustered iC3b coating a tumor cell. Several studies have shown the safety of β-glucans and the absence of side effects.
The targets for β-glucan-primed CR3 include any iC3b-opsonized host cell or microbial pathogen. Tumors appear to be frequently opsonized with IgM and/or IgG Abs and iC3b as the result of an ineffective humoral response and enhancement could occur with either vaccines or mAbs to tumor antigens. Cells infected with viruses or intracellular bacteria also often activate C, either because they have become activators of the alternative pathway or through Abs that activate the classical pathway of C. The common feature of target cell bound iC3b appears to explain the wide range of diseases that have been reported to respond to therapy with β-glucans (4,5).
Our data on mice have shown that resistance to β-glucan therapy corresponds to the absence of tumor cell-bound C3 and that the success of β-glucan therapy can be assured by antibodies to tumor antigens that enhance the target cell density of bound antibodies and C3 (6). Normal human and mouse sera contain low levels of Abs reactive with syngeneic or allogeneic tumor lines that activate complement, depositing iC3b onto tumors. Tumors implanted in mice became coated with IgM, IgG, and C3 and the absent C3 deposition on tumors in SCID mice was reconstituted with IgM or IgG isolated from normal syngeneic sera.
Rodent studies indicate that glucan supplements offset the increased risk of infection, either with or without stress association, mostly via augmentation of immunological activities, including cellular immunity (7). The defensive mechanisms of the lungs involve surface fluids (such as mucous and other material contained in the surface lining of the lungs; epithelial resources including cilia and mucous glands and alveolar macrophages; and immunocytologic reserves including the blood leukocytes and various immunoglobulins). Glucans were found effective in most of these cases (8,9), but the effects on mucosal immunity, thus far, have not been studied. At the same time, respiratory infections, particularly upper respiratory infections, are the highest-incidence acute illnesses in the developed world. According to the estimates, in the United States alone, the average adult has 2-to-4 colds per year and the average schoolchild 6-to-10 (10). Although patients with complications, such as bronchospasm or otitis media may benefit from antibiotic or inhaler treatment, medical science has little to offer for uncomplicated infections. Nevertheless, antibiotics are commonly prescribed, despite the well-established knowledge of little benefit. Clearly, there is a need for effective, safe, and inexpensive treatment of chronic respiratory problems. β-Glucan can be just the right solution.
Materials and methods
Protocol
A randomized, double-blind, placebo-controlled trial compared β-glucan #300 and placebo in children. Forty children (24 females, 16 males, age 8-12, average 10.7±2.3) from the sanatorium for respiratory diseases EDEL were enrolled in 4-week trial. The entire trial was conducted at the Sanatorium EDEL (Zlate Hory, Czech Republic) and the study was approved by the Ethics committee of the Public Health Institute and Sanatorium EDEL Czech Republic. This study was performed in agreement with Helsinki declaration (revised version 2000.09.01) and in full agreement of rules for clinical testing for the Czech Republic. Parental consent was given in all cases.
Subjects were randomly assigned to groups which were blinded to intervention. During the intervention period, subject consumed 100 mg/d of β-glucan or placebo. Both glucan and placebo capsules looked identical. Subjects were routinely evaluated by the medical staff.
Glucan
Yeast-derived insoluble glucan #300 were purchased from Transfer Point (Columbia, SC), this glucan is over 85% pure.
Tests
In all subjects we obtained saliva at the beginning of the study and at the endpoint of their stay in Sanatorium. We used identical times (between 8 and 9 AM) for sampeling, so the possible influence of circadian rhythms could be eliminated.
Saliva was collected using a commercial Salivette device (Sarstead, Orsay, France). A cotton swab was added into a sterile container and centrifuged at 2,000 g for 15 minutes and stored at –20 °C. We measured the levels of albumin, and C-reactive protein (CRP) in saliva using nephelometer Siemens BM II as suggested by the manufacturer. Lysozyme was measured using photometer Dynex MRX (The Microtiter Comp.) using egg lysozyme as a standard.
Statistical analysis
Statistical significance was evaluated by a pair t-test using a GraphPad Prism 502 software (GraphPad Software, USA).
Results
All children participating in our study are living at the same locality at Nothern Moravia, which is known for its extremely high level of polution. Only children diagnosed with repeated upper airways infections, chronic bronchitidis, allergies or asthma were used in this study.
All subjects were given identical food and were identically treated using climatotherapy and speleotherapy. In addition, the full medical examination was given at the begging and at the end of the trial.
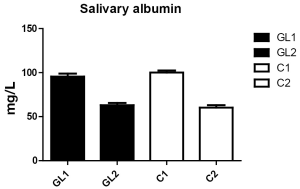
The level of albumin in saliva at the beginning of the trial was elevated in both groups, which corresponds to the actual clinical state of children living in heavily polluted areas. A month of treatment resulted in significant decrease of albumin levels in both groups (Table 1, Figure 1).

Full Table
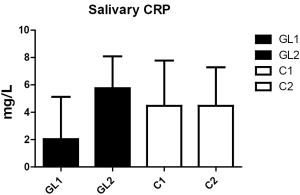
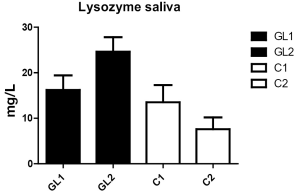
With respect to CRP, the levels did not significantly change during the study (Figure 2). However, the changes in lysozyme levels were very strong. In the glucan group, we observed significant increase (from 13.2 to 24.6 mg/L), whereas control group showed significant decrease (from 13.5 to 7.6 mg/mL; Figure 3).
Discussion
β-Glucan used in this study is one of the most studied glucans on the current market. Series of studies showed that it stimulates the cellular and humoral branches of immune system (11), protects against mercury poisoning (12), positively influences levels of cholesterol and blood sugar (13), inhibits cancer growth (14), and potentiates wound healing (15,16). In addition, these effects were similarly profound when administered orally or intraperitoneally (17).
This is the first placebo-driven clinical study to assess the effects of orally-administered glucan in children with chronic respiratory problems. As glucan can influence levels of secretory proteins in saliva (18), we decided to test the effects of orally-administered glucan on changes in some immunologically important proteins in saliva.
Albumin is a known indicator of inflammation (19), as albumin levels in saliva and other body fluids correspond to the degree of inflammation of mucose and are influenced by diffusion from capillary bed (20). Sanatorium for respiratory diseases is localized in area of extremely low pollution. As inflammation influenced by infection, environmental pollution and/or passive smoking increases diffusion of albumin into saliva (21,22), decrease in albumin levels in both tested groups corresponds with positive changes in atmospheric pollution during the tested interval. Another positive factor is the ending the influence of passive smoking (over 40% of children evaluated in this study was exposed to passive smoking by their parents).
Our findings of levels of C-reactive proteins in children’s saliva showed no significant results even after a month of treatment, with only slight increase in children with manifestation of infection of upper respiratory tract. We expect that higher levels of CRP at the beginning of the trial is influenced by passive smoking (21,23). Steady CRP levels suggest minimal effects of stress reaction and monitor positive effects of climatotherapy and speleotherapy in tested children (24).
The most important response to tested glucan was found in lysozyme levels. Monocytes are the source of lysozyme in saliva. Lysozyme represents an important component of innate non-immunoglobulin immunity with antimicrobial properties, ability to inhibit bacterial growth and metabolism (25). In addition, salivary levels of lysozyme can be influence by stress (26,27). However, glucan has been found to strongly increase the lysozyme production (28) on both protein and genomic level (29).
Salivary defense factors, including factors such as C-reactive protein and lysozyme, represent significant part of mucosal immunity, particularly in immunodeficient patients (30) and children prone to respiratory infections (31). In areas of heavy environmental pollution, the situation remains serious despite several compensatory actions including short-time moving to rural areas (32). Stimulation of immune system by well-established immunomodulator remains one of possible remedies. Our findings showed that short term oral administration of glucan significantly increased the salivary levels of CRP and lysozyme in children with chronic respiratory problems suggesting that this treatment stimulated mucosal immunity. From our results we can conclude that glucan administration might be considered as an inexpensive method in the treatment of chronic respiratory problems in children.
Acknowledgements
This work was supported by Technology Agency of the Czech Republic TACR TA 0202094.
Disclosure: The authors declare no conflict of interest.
References
- Novak M, Vetvicka V. Glucans as biological response modifiers. Endocr Metab Immune Disord Drug Targets 2009;9:67-75. [PubMed]
- Hamano K, Gohra H, Katoh T, et al. The preoperative administration of lentinan ameliorated the impairment of natural killer activity after cardiopulmonary bypass. Int J Immunopharmacol 1999;21:531-40. [PubMed]
- Ross GD, Baran JT, Allendorf DJ, et al. Newly identified function for the complement (C) system in regulating hematopoiesis and bone marrow reconstitution after radiation injury. Mol Immunol 2003;40:196-7.
- Ross GD, Vetvicka V, Yan J, et al. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology 1999;42:61-74. [PubMed]
- Thornton BP, Vĕtvicka V, Pitman M, et al. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J Immunol 1996;156:1235-46. [PubMed]
- Xia Y, Vetvicka V, Yan J, et al. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol 1999;162:2281-90. [PubMed]
- Davis JM, Murphy EA, Brown AS, et al. Effects of moderate exercise and oat beta-glucan on innate immune function and susceptibility to respiratory infection. Am J Physiol Regul Integr Comp Physiol 2004;286:R366-72. [PubMed]
- Novak M, Vetvicka V. Beta-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J Immunotoxicol 2008;5:47-57. [PubMed]
- Vetvicka V, Novak M. Biological action of β-glucan. In: Vetvicka V, Novak M, eds. Biology and Chemistry of Beta Glucan. Beerensteinerhof, Bussum: Bentham Science Publishers, 2011:10-8.
- Spector SL. The common cold: current therapy and natural history. J Allergy Clin Immunol 1995;95:1133-8. [PubMed]
- Vetvicka V, Vetvickova J. β-1,3-Glucan: Silver bullet or hot air? Open Glycoscience 2010;3:1-6.
- Vetvicka V, Vetvickova J. Effects of glucan on immunosuppressive actions of mercury. J Med Food 2009;12:1098-104. [PubMed]
- Vetvicka V, Vetvickova J. Effects of yeast-derived beta-glucans on blood cholesterol and macrophage functionality. J Immunotoxicol 2009;6:30-5. [PubMed]
- Yan J, Allendorf DJ, Brandley B. Yeast whole glucan particle (WGP) beta-glucan in conjunction with antitumour monoclonal antibodies to treat cancer. Expert Opin Biol Ther 2005;5:691-702. [PubMed]
- Vetvicka V, Vetvickova J. Physiological effects of different types of beta-glucan. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2007;151:225-31. [PubMed]
- Vetvicka V, Vetvickova J. β(1-3)-D-glucan affects adipogenesis, wound healing and inflammation. Orient Pharm Exp Med 2011;11:169-75.
- Vetvicka V, Vetvickova J. A comparison of injected and orally administered β-glucans. JANA 2008;11:42-8.
- Ramberg JE, Nelson ED, Sinnott RA. Immunomodulatory dietary polysaccharides: a systematic review of the literature. Nutr J 2010;9:54. [PubMed]
- Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004;17:432-7. [PubMed]
- Schenkels LC, Veerman EC, Nieuw Amerongen AV. Biochemical composition of human saliva in relation to other mucosal fluids. Crit Rev Oral Biol Med 1995;6:161-75. [PubMed]
- Azar R, Richard A. Elevated salivary C-reactive protein levels are associated with active and passive smoking in healthy youth: A pilot study. J Inflamm (Lond) 2011;8:37. [PubMed]
- Sköld CM, Blaschke E, Eklund A. Transient increases in albumin and hyaluronan in bronchoalveolar lavage fluid after quitting smoking: possible signs of reparative mechanisms. Respir Med 1996;90:523-9. [PubMed]
- Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem 2004;279:48487-90. [PubMed]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805-12. [PubMed]
- Bard E, Laibe S, Bettinger D, et al. New sensitive method for the measurement of lysozyme and lactoferrin for the assessment of innate mucosal immunity. part I: time-resolved immunofluorometric assay in serum and mucosal secretions. Clin Chem Lab Med 2003;41:127-33. [PubMed]
- Brown LR, Frome WJ, Wheatcroft MG, et al. The effect of Skylab on the chemical composition of saliva. J Dent Res 1977;56:1137-43. [PubMed]
- Soo-Quee Koh D, Choon-Huat Koh G. The use of salivary biomarkers in occupational and environmental medicine. Occup Environ Med 2007;64:202-10. [PubMed]
- Paulsen SM, Engstad RE, Robertsen B. Enhanced lysozyme production in Atlantic salmon (Salmo salar L.) macrophages treated with yeast beta-glucan and bacterial lipopolysaccharide. Fish Shellfish Immunol 2001;11:23-37. [PubMed]
- Wang YC, Chang PS, Chen HY. Differential time-series expression of immune-related genes of Pacific white shrimp Litopenaeus vannamei in response to dietary inclusion of beta-1,3-glucan. Fish Shellfish Immunol 2008;24:113-21. [PubMed]
- Kirstilä V, Tenovuo J, Ruuskanen O, et al. Salivary defense factors and oral health in patients with common variable immunodeficiency. J Clin Immunol 1994;14:229-36. [PubMed]
- Lehtonen OP, Tenovuo J, Aaltonen AS, et al. Immunoglobulins and innate factors of immunity in saliva of children prone to respiratory infections. Acta Pathol Microbiol Immunol Scand C 1987;95:35-40. [PubMed]
- Richter J, Pelech L. Immunological findings in groups of children after compensatory measures. Toxicol Lett 1996;88:165-8. [PubMed]


