
Muscle weakness in a S. pneumoniae sepsis mouse model
Introduction
Intensive care unit-acquired weakness (ICU-AW) is an important complication of critical illness. It is caused by dysfunction or structural damage of nerves (critical illness polyneuropathy, CIP), muscles (critical illness myopathy, CIM) or both (critical illness neuromyopathy, CINM). ICU-AW is characterized by diffuse weakness of both limb and respiratory muscles (1,2). Selective myosin filament loss is characteristic of CIM and can be seen within 5 days after ICU admission (3).
The main risk factors for ICU-AW are sepsis, the systemic inflammatory response syndrome (SIRS) and multiple organ failure (4). The pathophysiology is complex and not completely understood, which seriously hampers the development of potential therapeutic possibilities for ICU-AW.
To further unravel the pathophysiology of ICU-AW, an animal model is needed. Several animal models have been used to study muscle dysfunction in ICU-AW, but all these models have their limitations. Some frequently used models are far away from the human ICU situation, e.g., the model of muscle denervation and dexamethasone treatment (5,6), or have low consistency and reproducibility, e.g., the cecal ligation and puncture (CLP) model to induce sepsis (7). Others mimick ICU conditions more closely, but are very expensive and time-consuming because they need continuous monitoring, e.g., porcine (8) or rat (9) models with several days of mechanical ventilation. Another important limitation of the existing models is that in vivo strength measurements have been scarcely performed, whereas decreased muscle strength is a prerequisite for the diagnosis of ICU-AW in humans (10). Only surrogate markers for muscle strength like electrophysiological studies and contractility measurements have been used (5,8). The translatability of these models to ICU-AW in humans is therefore uncertain.
We aimed to use a well-known and easily applicable S. pneumoniae sepsis model to induce ICU-AW in mice (11,12). The primary objective of this study was to investigate whether this sepsis model could serve as an ICU-AW model that more resembles the human disease, using in vivo muscle strength measurements as our primary endpoint.
Methods
Animals and ethical approval
A total of 36, 8–10 weeks old male, specific-pathogen-free, C57BL/6J mice were obtained from Charles River. To acclimatize, mice were housed in groups of four in individually ventilated cages for nine days before the start of the experiment. Food and water were available ad libitum and a 12:12 hour light-dark cycle was retained.
All experiments conformed the Dutch Experiments on Animals Act for the care and use of animals and were approved by the Institutional Animal Care and Use Committee of the Academic Medical Center, Amsterdam, the Netherlands (permit number: 102904).
This manuscript was drafted in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (13).
Induction of pneumonia
Mice were inoculated intranasally with S. pneumoniae as described elsewhere (14). In short, S. pneumoniae serotype 3 (American Type Culture Collection 603) were cultured in Todd-Hewitt broth at 37 °C in 5% CO2 for 4 h to a mid-logarithmic phase, harvested by centrifugation at 4,000 rpm for 10 min, and washed twice in sterile isotonic saline. Bacteria were then resuspended in sterile isotonic saline and diluted to a concentration of ~4×106 colony-forming units (CFU)/mL, as determined by plating serial 10-fold dilutions onto sheep-blood agar plates. Mice were lightly anesthetized by inhalation of isoflurane and 50 µL (~2×105 CFU) was inoculated intranasally.
Experimental procedures
Experimental procedures of experiment 1 and 2 are presented in Figure 1. In experiment 1, mice were assigned to three groups (random per cage): Blank group (n=4), Control1 group (n=4) and S. pneumoniae and antibiotics group (AB24, n=8). Mice of the Blank group did not receive any inoculation under isoflurane nor any intraperitoneal injections. At the start of the experiment (t=0), mice of the AB24 group were inoculated with S. pneumoniae, while the Control1 group received 50 µL of sterile isotonic saline intranasally. To mimic human sepsis treatment, improve survival and maintain fluid volume status, mice received intraperitoneal injections with antibiotics in saline. Mice of the AB24 and Control1 group received ceftriaxone (Fresenius Kabi, Den Bosch, the Netherlands), 20 mg/kg and 10 µL/g body weight at 24, 48 and 72 hours after inoculation. Body weight and discomfort, such as reduced locomotor activity, were measured at least twice daily.
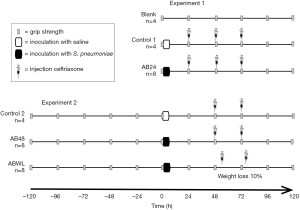
In experiment 2, mice were assigned to three groups (random per cage): Control2 group (n=4), S. pneumoniae and antibiotics group (AB48, n=8) and S. pneumoniae and antibiotics at weight loss group (ABWL, n=8). The first antibiotic treatment was either delayed for another 24 hours or administered as soon as mice lost 10% of their body weight (compared to the maximal weight until that time point). Also, mice received two instead of three recurrent injections of ceftriaxone (20 mg/kg and 10 µL/g body weight). Inoculation with S. pneumoniae or saline was performed as in experiment 1. At 48 and 72 hours after inoculation, mice of the Control2 and AB48 group received an intraperitoneal injection with ceftriaxone. Mice of the ABWL group received ceftriaxone as soon as they lost 10% of their body weight and 24 hours thereafter. Body weight and discomfort was measured at least three times daily.
Grip strength testing
Fore limb grip strength was measured daily (~9.00 AM) by an experienced investigator (EW) using a grip strength meter with metal grid (Bioseb, France) as previously described (15). At each time point, three grip strength measurements were taken, of which the average result was used for analysis. Grip strength was normalized for concomitant body weight (16).
In experiment 2, grip strength measurements were taken daily from 120 hours before to 120 hours after inoculation.
Electrophysiological recordings
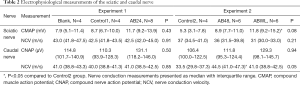
At the end of the experiment, at t=120 hours after inoculation, mice were anesthetized by inhalation of isoflurane. Tail and hind limbs were strapped to a board and a heating pad maintained body temperature. The sciatic and caudal nerves were studied on one side on a Viking III EMG machine (Nicolet, Madison, USA) by insertion of stimulating and recording monopolar needle electrodes followed by supramaximal stimulation. Compound muscle action potentials (CMAPs) amplitudes (peak to peak) of the sciatic nerve were recorded and motor nerve conduction velocities (NCVs) over the segment between the ankle and the sciatic notch were calculated. For studies of the caudal nerve, compound nerve action potentials (CNAPs) amplitudes (baseline to negative peak) were recorded and NCVs were calculated.
Euthanasia
After the electrophysiological recordings, mice were euthanized by an intraperitoneal injection of a mix of 126 mg/kg ketamine (Nimatek, Eurovet Animal Health BV, The Netherlands), 0.1 mg/kg dexmedetomidine (Dexdomitor, Orion pharma, Finland), and 0.5 mg/kg atropine (Pharmachemie BV, The Netherlands) in sterile saline followed by dissection of the carotid artery.
Lung and spleen were harvested for determination of bacterial outgrowth. Fresh muscle strips, dissected from the excised diaphragm, were placed overnight at 5 °C in relaxing solution (for composition, see below) containing 1% Triton X-100 to permeabilize the membranes. Subsequently, the specimens were washed overnight with relaxing solution, then placed in a 50% glycerol/relaxing solution (vol/vol), and stored at −20 °C until further use.
Bacterial outgrowth
Lung and spleen were homogenized in four volumes of sterile saline with a tissue homogenizer. Serial ten-fold dilutions of the homogenates were plated on sheep-blood agar plates and bacteria were allowed to grow at 37 °C.
Diaphragm fiber contractility measurements and myosin-actin ratio measurements
As it is not possible to measure diaphragm strength in awake mice and in vivo electrophysiological recordings of diaphragm in mice are hard to perform, we investigated diaphragm strength ex vivo in the second experiment. Fiber contractile measurements and experimental protocols were performed according to previously described methods with minor modifications (17).
Small bundles of fibers (100–150 μm in diameter) were isolated from the diaphragm samples using micro forceps. The fiber ends were attached to aluminum-foil clips and mounted on a muscle-fiber apparatus (Aurora Scientific, Aurora, Ontario, Canada), which was placed on top of an inverted microscope. One end of the fiber bundle was attached to a force transducer (model 403A, Aurora Scientific), whereas the other end was attached to a servomotor (315C, Aurora Scientific). Preparations that appeared damaged during microscopic examination were excluded from the study. The number of excluded fiber bundles did not differ per group. All measurements were performed at 20 °C.
The composition of relaxing solution (total ionic strength of 180 mM) consisted of 5.89 mM Na2ATP, 6.48 mM MgCl2, 40.76 mM K-propionate, 100 mM BES, 6.97 mM EGTA and 14.5 mM CrP with sufficient KOH to adjust the pH to 7.1. The negative logarithm of the free Ca2+ concentration (pCa) of the relaxing solution was set at 9.0, whereas the activating solution was set at pCa 4.5.
While in relaxing solution, sarcomere length was set at 2.5 µm using a fast Fourier transformation on a region of interest on the real-time camera image. Muscle fiber length and thickness (in both x-y and x-z direction) were measured using the live camera image. The cross-sectional area (CSA) was calculated from the thickness measurements, assuming that the cross section of the fiber bundle is ellipsoidal. All active forces were measured at a sarcomere length of 2.5 µm and are expressed as tension (force per CSA).
The fiber bundle was placed for 1 minute in pre-activating solution before maximal isometric force was measured upon placement of the fiber in activating solution (pCa 4.5). The rate constant of force redevelopment (ktr) was measured during maximal activation (pCa 4.5) by rapidly releasing the fiber by 30% of its original length, followed by a quick restretch to its original length. This release detaches all myosin heads attached to actin, and subsequently force redevelops. The ktr was determined by fitting a double exponential through the force redevelopment curve (note that only the fast rate constant is reported as this is considered to reflect cross-bridge cycling kinetics). Active stiffness was determined during maximal activation (pCa 4.5) by imposing small length perturbations (0.3%, 0.6%, 0.9% of initial length) on the fiber bundle resulting in a quick force response. The tension change (∆T) was plotted as a function of the length change (∆L). Active stiffness was derived from the slope of the fitted line and is a measure to estimate the number of cycling cross-bridges. The ratio of maximal tension and active stiffness reflects the force generated per cross-bridge (18).
Finally, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used to determine the myosin-actin ratio in the muscle fiber preparations that we used in our contractility experiments. Muscles fibers were de-natured by boiling at 80 °C for 2 min in SDS sample buffer. Samples were loaded on a 7–15% acrylamide gel. The gels were run for 3 h at 15 °C and a constant voltage of 275 V. Finally, the gels were stained with sypro-Ruby, scanned, and the myosin heavy chain and actin protein bands were analyzed with AIDA Image Analyzer software (Raytest Isotopenmessgeräte GmbH, Straubenhardt, Germany).
Experimental outcomes
The primary outcome was a decrease in grip strength, and secondary outcomes were differences between experimental and control groups in electrophysiological recordings, diaphragm muscle fiber strength and myosin-actin ratio of the diaphragm.
Power calculation and statistical analysis
We chose the number of animals per group on pragmatic grounds, because no previous data on grip strength in this animal model were available to support a power calculation.
Unless otherwise stated, continuous variables are presented as medians with interquartile range (IQR). Kruskal-Wallis test was used to compare continuous variables between the three groups in each experiment. The experimental groups were compared to the control groups by use of the Mann-Whitney U test. Weight loss was calculated by the percentage of difference between the weight and the maximal weight until that time point.
For the analysis of diaphragm muscle contractility normal distribution was tested. If data was normally distributed multilevel analysis to correct for non-independence of successive measurements per animal (MLwiN, 2.02.3; Center for Multilevel Modelling, Bristol, UK) was used (17,18).
Statistical significance was defined as P<0.05. Analyses were done using R (version: 3.02; R Foundation for Statistical Computing, Vienna, Austria).
Results
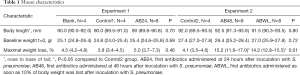
Baseline bodyweight and length were not significantly different between the various experimental groups for both experiments (Table 1).
Full table
Clinical illness severity and bacterial outgrowth
Mice of the AB24 group of experiment 1 showed slightly reduced locomotor activity. Maximal weight loss during the experiment, as a measure of illness severity, was 5.0% (median, IQR 3.7–7.3) in the AB24 group and was equal to the Control1 and Blank group (P=0.46; Table 1 and Figure 2A).
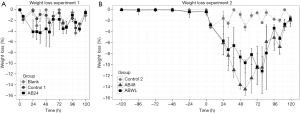
Because mice did not develop severe illness in experiment 1, which was possibly prevented by early and recurrent administration of antibiotics, in experiment 2 the first antibiotic treatment was either delayed for another 24 hours or administered as soon as mice lost 10% of their body weight. Furthermore, antibiotics were administered twice instead of three times. As a result, mice in the AB48 and ABWL group became more severely ill, represented by considerable reduced locomotor activity and a significant weight loss of 15.2% (IQR 11.6–17.0) in the AB48 group and 14.2% (IQR 12.8–15.5) in the ABWL group (P<0.05 compared to the Control2 group [4.1% (IQR 3.5–4.6); Table 1, Figure 2B]. In the ABWL group the first administration of antibiotics was at 47 hours (median, IQR 31–68).
Two mice (one in the AB48 and one in the ABWL group) died before the end of the experiment and two other mice (one in the AB48 and one in the ABWL group) had a hunched posture and severely decreased locomotor activity and had to be euthanized before the end of the experiment according to predefined humane endpoints.
In experiment 1, homogenates of lung and spleen showed no bacterial outgrowth. In experiment 2, five of six lung homogenates of the AB48 group and all six spleen homogenates showed bacterial outgrowth. In the ABWL group all of six lung and three of six spleen homogenates showed bacterial outgrowth.
Grip strength
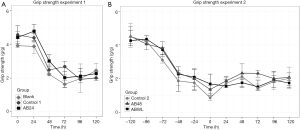
Results of grip strength measurements over time are presented in Figure 3. In experiment 1, grip strength declined in all groups, with the biggest drop within the first 72 hours after inoculation. No differences were seen between the experimental and control groups. Also, in the Blank group, the group that did not receive any interventions such as inoculation or injections, grip strength declined. Because of this decline in grip strength in the first few days in all groups of experiment 1, we chose to incorporate a grip strength training phase in experiment 2, starting five days before inoculation. Despite the fact that mice in the AB48 and ABWL group became more severely ill, no differences were seen between the experimental and control groups during 5 days of follow-up.
Electrophysiological recordings
Results of electrophysiological recordings are presented in Table 2. No differences in electrophysiological recordings were found between the groups in experiment 1. In experiment 2, CMAPs of the sciatic nerve were higher in the ABWL group compared to the Control2 group. NCV of the caudal nerve was higher in the AB48 group compared to the Control2 group.
Full table
Muscle fiber contractility
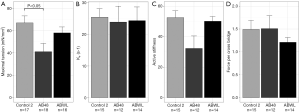
Muscle fiber contractility was measured in diaphragm samples of nine mice, three of each group of experiment 2. Maximal tension (force per CSA) was lower in the AB48 group compared to the Control2 group (41.1 versus 65.8 nM/mm2, P=0.0084) (Figure 4A). A reduction in tension in single muscle fibers can be explained by one or more of the following factors: the number of available cross bridges, the fraction of strongly bound cross-bridges and the force per cross-bridge (19). There were no changes in the rate constant of force redevelopment (ktr) indicating that the fraction of strongly bound cross bridges was unaltered (Figure 4B). The force generated per cross bridge was also not different (i.e., the ratio of maximal tension and active stiffness) (Figure 4C,D). This indicates that the lower maximal tension in the AB48 group is caused by a limited number of available cross bridges.
Myosin-actin ratio in diaphragm
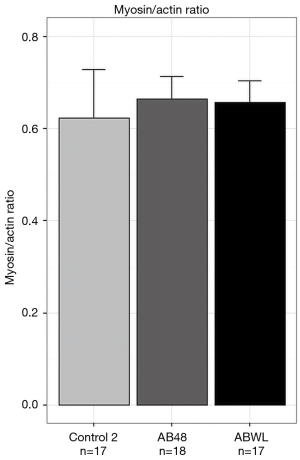
Myosin-actin ratio was determined in all diaphragm fiber bundle of the contractility measurements. There were no differences in myosin-actin ratio between the groups (Figure 5).
Discussion
Grip strength and electrophysiological measurements
In our S. pneumoniae model experiments, grip strength decreased, but there was no difference between control and experimental groups. In the first experiment, we found a decline in grip strength within the first 72 hours in all groups, including the mice that did not underwent any intervention. We also saw this decline in a previous experiment with an E. coli infection (15). In fact, a risk for learning bias has been described with frequent grip strength testing (20). Therefore, we included a training phase in our subsequent experiment. We started grip strength testing five days before inoculation in an attempt to diminish this effect. In this experiment, again a decline in grip strength was seen during the training phase. After inoculation strength remained stable without any differences between the groups. Because of this probable learning effect grip strength testing may not be the best in vivo method to detect acute muscle weakness in awake mice. Other in vivo methods could be considered, such as the rotarod test or inverted screen, although learning bias can also be a problem with the rotarod test (21).
Besides grip strength we also investigated peripheral nerve or muscle involvement by electrophysiological recordings. The most common findings in nerve conduction studies in ICU-AW patients are reduced CMAP amplitudes (in CIM and CIP) and/or SNAP amplitudes (in CIP) and normal or slowed conduction velocities (1,22). Nerve conduction studies in our experiments did not show abnormalities as can be seen in ICU-AW patients. CMAP amplitudes of the sciatic nerve in the second experiment were even higher in the S. pneumoniae groups compared to the control group. We do regard this as a co-incidence, which may be due to the small sample size. Because of their possible harm, electrophysiological measurements were only done at the end of the experiment. Reversible changes may thus have been missed. We did not perform needle electromyography to detect abnormal spontaneous activity, which may be more sensitive than nerve conduction studies to detect muscle damage, either primary or secondary to nerve damage (23).
Diaphragm contractility and myosin-actin ratio
Because the diaphragm is also effected by ICU-AW and diaphragm weakness is a major cause for prolonged mechanical ventilation, we also investigated the diaphragm from severely ill mice (24,25). Besides mechanical ventilation (26), inflammation (27) also causes diaphragmatic weakness.
Muscle fiber contractility measurements of the diaphragm showed a reduction of maximal tension in mice of the AB48 group compared to controls. This was explained by a limited number of available cross bridges. However, myosin-actin ratio was not different between these groups. This suggests that, instead of selective myosin loss, which is described in ICU-AW, part of the contractile material is damaged and is therefore not functional anymore. This is in line with findings from diaphragm fibers of critically ill patients who were mechanically ventilated for a median 88 hours prior to biopsy (28). Individual diaphragm fibers of these patients were severely weakened, both by atrophy and by dysfunction of the remaining contractile proteins, without evidence for selective myosin loss.
Suitability of this model to study ICU-AW
This model does not capture the full clinical spectrum of ICU-AW as seen in patients. We found no evidence of limb muscle weakness. On the other hand, diaphragmatic weakness was found.
Limb muscle function was assessed with grip strength and nerve conduction studies. As these assessments did not show abnormalities, this model does not resemble patients with ICU-AW. However, we cannot rule out that more sensitive methods such as needle electromyography, and muscle and nerve biopsies, may reveal evidence for limb muscle and peripheral nerve involvement.
Although mice were severely ill and several of the animals died during the experiment, the threshold needed to develop limb weakness may not have been reached in this model. Mice recovered quickly as was also indicated by the restored body weight. In our second experiment, maximum weight loss was reached after 2–3 days and at the end of the experiment body weight was almost on baseline level. However, since 25% of mice in the experimental groups died or had to be euthanized due to too severe illness, further postponing or reducing the antibiotic treatment does not seem to be an option. Pathogens with a more prolonged/chronic critical illness may be used to induce sepsis, or mechanical ventilation or sedation could be added to the model to introduce a second hit, but the latter two would impair in vivo strength measurements.
Contractility measurements of the diaphragm showed weakness in the group of mice with the most severe weight loss. Previous animal studies have shown that the diaphragm is more susceptible to systemic inflammation; i.e., diaphragm muscle contractility was decreased, while limb muscle contractility was preserved (29,30). This S. pneumoniae model may be used to further study inflammation induced diaphragm weakness.
Limitations of this study
In addition to the limitations already stated above, this study has some other limitations. We did not perform a power calculation for this study, because no previous data on grip strength in this animal model were available. Secondly, we used different methods to detect weakness in diaphragm and limb muscles. We performed ex vivo contractility measurements of the diaphragm, but we did not perform these measurements in limb muscle. We can therefore not rule out that contractility measurements may have shown changes as well in limb muscles, despite a lack of change in grip strength and electrophysiological measurements. At last, we only used young mice in our experiments.
Conclusions
Although mice became severely ill, this S. pneumoniae mouse model showed no weakness with in vivo strength measurements and electrophysiological studies as seen in humans with ICU-AW. As such, this model did not fulfill our predefined requirements. However, weakness of the diaphragm was found ex vivo, and as weaning from a ventilator is a major issue in human ICU-AW this model may be used to study inflammation induced diaphragmatic weakness.
Acknowledgements
The authors thank K van der Sluijs and J. Buchner for their technical assistance during the animal experiments.
Funding: L Wieske is supported by a personal grant from the Netherlands Organization for Health Research and Development [ZonMw-AGIKO grant (project number 40-00703-98-11636)].
Footnote
Conflicts of Interest: Prof. IN van Schaik received departmental honoraria for serving on scientific advisory boards and a steering committee for CSL-Behring. The other authors have no conflicts of interest to declare.
Ethical Statement: All experiments conformed the Dutch Experiments on Animals Act for the care and use of animals and were approved by the Institutional Animal Care and Use Committee of the Academic Medical Center, Amsterdam, the Netherlands (permit number: 102904).
References
- Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med 2009;37:S299-308. [Crossref] [PubMed]
- Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med 2014;370:1626-35. [Crossref] [PubMed]
- Wollersheim T, Woehlecke J, Krebs M, et al. Dynamics of myosin degradation in intensive care unit-acquired weakness during severe critical illness. Intensive Care Med 2014;40:528-38. [Crossref] [PubMed]
- Stevens RD, Dowdy DW, Michaels RK, et al. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med 2007;33:1876-91. [Crossref] [PubMed]
- Rich MM, Pinter MJ. Sodium channel inactivation in an animal model of acute quadriplegic myopathy. Ann Neurol 2001;50:26-33. [Crossref] [PubMed]
- Mozaffar T, Haddad F, Zeng M, et al. Molecular and cellular defects of skeletal muscle in an animal model of acute quadriplegic myopathy. Muscle Nerve 2007;35:55-65. [Crossref] [PubMed]
- Rittirsch D, Hoesel LM, Ward P. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol 2007;81:137-43. [Crossref] [PubMed]
- Aare S, Radell P, Eriksson LI, et al. Role of sepsis in the development of limb muscle weakness in a porcine intensive care unit model. Physiol Genomics 2012;44:865-77. [Crossref] [PubMed]
- Llano-Diez M, Gustafson A-M, Olsson C, et al. Muscle wasting and the temporal gene expression pattern in a novel rat intensive care unit model. BMC Genomics 2011;12:602. [Crossref] [PubMed]
- Fan E, Cheek F, Chlan L, et al. An Official American Thoracic Society Clinical Practice Guideline: The Diagnosis of Intensive Care Unit-acquired Weakness in Adults. Am J Respir Crit Care Med 2014;190:1437-46. [Crossref] [PubMed]
- Van Den Boogaard FE, Brands X, Schultz MJ, et al. Recombinant human tissue factor pathway inhibitor exerts anticoagulant, anti-inflammatory and antimicrobial effects in murine pneumococcal pneumonia. J Thromb Haemost 2011;9:122-32. [Crossref] [PubMed]
- Schouten M, van ’t Veer C, van den Boogaard FE, et al. Therapeutic recombinant murine activated protein C attenuates pulmonary coagulopathy and improves survival in murine pneumococcal pneumonia. J Infect Dis 2010;202:1600-7. [Crossref] [PubMed]
- Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. [Crossref] [PubMed]
- van der Poll T, Keogh C V, Buurman WA, et al. Passive immunization against tumor necrosis factor-alpha impairs host defense during pneumococcal pneumonia in mice. Am J Respir Crit Care Med 1997;155:603-8. [Crossref] [PubMed]
- Witteveen E, Hoogland ICM, Wieske L, et al. Assessment of intensive care unit-acquired weakness in young and old mice: An E. coli septic peritonitis model. Muscle Nerve 2016;53:127-33. [Crossref] [PubMed]
- Maurissen JPJ, Marable BR, Andrus AK, et al. Factors affecting grip strength testing. Neurotoxicol Teratol 2003;25:543-53. [Crossref] [PubMed]
- Manders E, de Man FS, Handoko ML, et al. Diaphragm weakness in pulmonary arterial hypertension: role of sarcomeric dysfunction. Am J Physiol Lung Cell Mol Physiol 2012;303:L1070-8. [Crossref] [PubMed]
- Manders E, Bonta P, Kloek J, et al. Reduced force of diaphragm muscle fibers in patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2016;311:L20-8. [Crossref] [PubMed]
- Geiger PC, Cody MJ, Macken RL, et al. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol (1985) 2000;89:695-703. [Crossref] [PubMed]
- DeLuca A: Use of grip strength meter to assess the limb strength of mdx mice. 1–11 available: http://www.treat–eu/downloads/, 2014. Available online: http://www.treat-nmd.eu/downloads/file/sops/dmd/MDX/DMD_M.2.2.001.pdf.
- Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci 2009;10:519-29. [Crossref] [PubMed]
- Lacomis D. Electrophysiology of neuromuscular disorders in critical illness. Muscle Nerve 2013;47:452-63. [Crossref] [PubMed]
- Khan J, Harrison TB, Rich MM, et al. Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology 2006;67:1421-5. [Crossref] [PubMed]
- Maher J, Rutledge F, Remtulla H, et al. Neuromuscular disorders associated with failure to wean from the ventilator. Intensive Care Med 1995;21:737-43. [Crossref] [PubMed]
- De Jonghe B, Bastuji-Garin S, Sharshar T, et al. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med 2004;30:1117-21. [Crossref] [PubMed]
- Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011;183:364-71. [Crossref] [PubMed]
- Reid MB, Lannergren J, Westerblad H. Respiratory and Limb Muscle Weakness Induced by Tumor Necrosis Factor-alpha: Involvement of Muscle Myofilaments. Am J Respir Crit Care Med 2002;166:479-84. [Crossref] [PubMed]
- Hooijman PE, Beishuizen A, Witt CC, et al. Diaphragm Muscle Fiber Weakness and Ubiquitin-Proteasome Activation in Critically Ill Patients. Am J Respir Crit Care Med 2015;191:1126-38. [Crossref] [PubMed]
- Divangahi M, Matecki S, Dudley RWR, et al. Preferential diaphragmatic weakness during sustained Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med 2004;169:679-86. [Crossref] [PubMed]
- Li X, Moody MR, Engel D, et al. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 2000;102:1690-6. [Crossref] [PubMed]


