Altering cough reflex sensitivity with aerosolized capsaicin paired with behavioral cough suppression: a proof-of-concept study
Introduction
Chronic cough (CC), defined as a cough lasting more than 8 weeks, is one of the most common complaints for which patients seek medical care (1-6). Patients who suffer from CC have significant impairments to quality of life and experience psychological and social stress (7-9). Despite extensive testing and medication trials, approximately 10–20% of patients who suffer from CC do not respond to medical treatment, and are said to have refractory CC (RCC) (1,10-14). Research has shown that the underlying cause of RCC in many patients is hypersensitivity of afferent sensory neurons in the mucosal lining of the airway, in particular the transient receptor potential vanilloid type I receptor (TRPV1) on C-fibers is over-expressed (11,15-18). There is evidence for hypersensitization in the central nervous system as well (19-21). This hypersensitivity results in the elicitation of cough by a variety of stimulants including chemical fumes, perfumes, cold air, and even talking, laughing, or singing (11,15-18,22). This specific subset of cough is now commonly termed cough hypersensitivity syndrome (CHS) (11,16,18,23). Although the exact cause of CHS is unknown, it is thought to be due to a neuroplastic mechanism in response to airway inflammation, presumably from a cough-inducing illness such as an upper respiratory infection. For an unknown reason, the hypersensitivity does not resolve spontaneously, and is then perpetuated by the continual coughing (11,15,16). Evidence suggests that a similar mechanism is responsible for chronic neuropathic pain, and chronic rhinitis, both of which also involve TRPV1 receptors (24-26).
Several peer-reviewed studies, including two randomized controlled trials, demonstrate efficacy of behavioral treatment for RCC (10,27-35). Although the name of the therapy, and what professional delivers it (e.g., speech-language pathologist, physiotherapist, respiratory therapist), varies throughout the literature, every behavioral program focuses heavily on cough suppression strategies (27-36). Consequently, we have chosen the term behavioral cough suppression therapy (BCST) to describe this treatment (10). The exact mechanism responsible for the success of BCST is unclear, but studies showing an increase in capsaicin cough reflex threshold following successful treatment with BCST (27,32,34) suggest BCST stimulates a reduction in sensitivity via the Use-It-Or-Lose-It principle of neuroplasticity (27,37,38). In other words, avoidance of cough, in the presence of an afferent signal eliciting an urge-to-cough (UTC), is thought to result in down modulation of the afferent signal and subsequent reduced sensitivity.
Although a majority of patients with RCC benefit from BCST (10,27,28,31,34), some patients are not able to suppress their cough in the presence of environmental stimuli, presumably because they have such severe hypersensitivity. In our experience, these patients do not respond well to BCST. We hypothesize these patients would benefit from BCST if they could be exposed to a cough stimulant strong enough to elicit an UTC, but weak enough to allow for successful cough suppression. Aerosolized capsaicin is an ideal cough stimulant to test this hypothesis because of its established safety and reproducibility (39-42) as well as the ability to dilute it to any concentration.
The purpose of this study was to determine feasibility and establish proof-of-concept of programmatically decreasing cough sensitivity by pairing cough suppression strategies with inhalation of aerosolized capsaicin given in gradually increasing doses, across repeated treatment sessions. If effective, this treatment may benefit patients with RCC who do not respond to BCST because they are too sensitive to suppress their cough in the presence of uncontrollable environmental stimuli. The study was approved by The University of Montana Institutional Review Board (#249-17) on November 28, 2017 for testing on healthy individuals. Written informed consent was obtained from each participant.
Methods
Design and participants
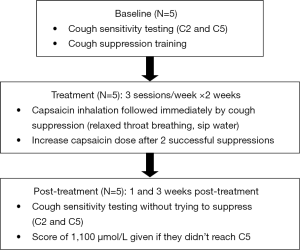
Five adults, 2 men and 3 women (mean age =25.8; SD =4.79), from University of Montana’s Communicative Sciences and Disorders department enrolled and completed this quasi-experimental, non-randomized, single cohort proof-of-concept study. All participants were healthy individuals with no current signs of upper respiratory infection or illness, without chronic respiratory conditions [asthma, chronic obstructive pulmonary disease (COPD), etc.], and were not current smokers. Subjects were given a $25 gift card for participating. The study consisted of baseline capsaicin cough sensitivity testing, 5–6 cough desensitization treatment sessions across a 2-week period, and two post-treatment cough sensitivity testing sessions, administered at 1 and 3 weeks post-treatment. A flowchart of the methods is provided in Figure 1.
Baseline cough sensitivity testing
Single-breath, dose-response, capsaicin cough sensitivity testing, as described in the literature (39-42), was completed at baseline and 1 and 3-week post-treatment. Spirometry was completed before and after each testing session and each treatment session. Participants wore a nose clip during spirometry. Forced vital capacity (FVC), forced expiratory volume in the first second after a maximum inhale (FEV1), forced expiratory flow at 50% of the FVC (FEV 50%) and FEV1/FVC were recorded and a minimum of two measures were taken to ensure reliable results. The higher value of each of the two trials was recorded.
Pharmaceutical-grade capsaicin, purchased from Sigma-Aldrich, was used for cough sensitivity testing and treatment sessions. A pharmacist in the pharmaceutical sciences department at The University of Montana dissolved 50 mg of capsaicin in 1.637 mL ethanol to make a 0.1 mol/L stock solution (43) We chose not to add Tween when making the stock solution due to its foul taste and the work of Costanzo, Yost, & Davenport (2014) who demonstrated that dissolving in at least 10% ethanol provided sufficient solubility (43).
The Koko DigiDoser with nebulizer was used for delivery of the aerosolized capsaicin. The dosimeter was calibrated each day, prior to testing. Participants were instructed to take full breaths in and out of the nebulizer prior to delivery of the aerosolized capsaicin. They were coached to complete a single inhale once they had received the capsaicin dose, and then remove the mouthpiece. They were instructed not to talk for 15 seconds following dose administration, and not to attempt to suppress any UTC, but rather to allow their body to respond naturally. Participants were first given one or two trials of saline to familiarize with the sensation, and to ensure accurate execution of the procedures. The lowest concentration of capsaicin (3.91 µmol/L) was given first. At least 30 seconds passed before each subsequent dose was given, to allow for recovery. The dose was increased until the participant coughed five or more times within 15 seconds of dose delivery. Doses that elicited two coughs (C2) and five coughs (C5) were recorded. Testing ceased when participants met the C5 threshold, or following administration of the maximum dose of 1,000 µmol/L.
Following baseline testing, participants were instructed in cough suppression techniques, specifically relaxed-throat breathing and cough suppression effortful swallow (44). They were instructed to practice 10 cycles of relaxed-throat breathing, twice each day prior to and throughout the treatment phase.
We chose not to add Tween when making the stock solution due to its foul taste and the work of Costanzo, Yost, & Davenport (2014) who demonstrated that dissolving in at least 10% ethanol provided sufficient solubility (43).
Treatment sessions
The first author made capsaicin dilutions, as needed, during the treatment phase. Dilutions were made no more than 3 days prior to each session and were stored in opaque vials at 2 °C. Treatment sessions were completed 2–3 times per week for the 2 weeks following baseline testing. Each session lasted 30–40 minutes. Each participant completed five or six sessions, depending on availability.
Treatment sessions began with spirometry, as described above. Participants were then exposed to increasing doses of aerosolized capsaicin, through the Koko DigiDoser. They were coached to suppress their cough after each exposure. Participants were instructed to complete their inhale after receiving the capsaicin, then forcefully blow out with tightly pursed lips, followed by cycles of relaxed-throat breathing, until the UTC dissipated. One to two saline trials were conducted initially to ensure accurate execution of the procedures. The first capsaicin dose given was two-concentration levels below each participant’s baseline C2 threshold. The capsaicin concentration was increased when each participant was able to receive 2–3 exposures of the same dose without coughing. Each increase of capsaicin was a doubling dose up to 500 µmol/L. Two concentrations between 500 and 1,000 µmol/L (i.e., 625 and 750 µmol/L) were used to minimize the large increase between 500 and 1,000 µmol/L. Participants were typically exposed to 3–4 different doses of capsaicin during each 30–40 minutes session, depending on their success with cough suppression. Subsequent sessions began 1–2 levels below the prior session’s highest successfully suppressed concentration. In addition to relaxed-throat breathing, participants were permitted to sip water or use a cough-suppression hard swallow to help suppress their cough, if desired, although no participant used either of these strategies. Participants were given as much time as needed prior to each subsequent exposure to ensure any burning or tingling sensation in the throat had completely dissipated. This rarely took more than 1–2 minutes. Participants were intermittently asked, during and between treatment sessions, about potential adverse effects from the repeated exposure to capsaicin.
Post-treatment cough sensitivity testing
Post-treatment cough sensitivity testing was completed 1 and 3 weeks following the final treatment session. Fresh capsaicin dilutions were used for each testing session. The same procedure was used as during baseline sensitivity testing except for the insertion of 1–3 random saline trials to control for an anticipation effect. If the participant did not reach the C2 or C5 threshold at 1,000 µmol/L, they were assigned a score of 1,250 µmol/L, for the purpose of statistical analyses.
Data analysis
The data were analyzed using IBM Statistical Package for the Social Sciences (SPSS) statistical analysis software. The Wilcoxon Signed Rank Test was used to analyze differences between baseline and 1 week and baseline and 3 weeks post-treatment on logC2 and logC5 values.
Results
Spirometry measures before and after cough sensitivity testing and before and after each treatment sessions were normal. One participant reported a lingering taste of pepper in his mouth for a few hours following treatment sessions. No other adverse effects were reported for the duration of the study.
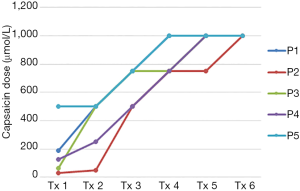
Baseline cough threshold values for each participant are shown in Table 1. Each participant demonstrated a gradual increase in maximum capsaicin dose suppressed during each treatment session, with each successfully suppressing at 1,000 µmol/L by the final treatment session (Figure 2). C2 was greater than baseline in 4 of 5 participants at 1 week post-treatment and in 3 of 5 participants at 3 weeks post-treatment. C5 was greater than baseline in all 5 participants at both post-treatment time points. C2 and C5 scores for each participant at baseline and during the post-treatment phase are shown in Table 1 and Figures 3,4.
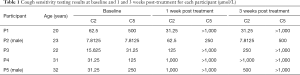
Full table
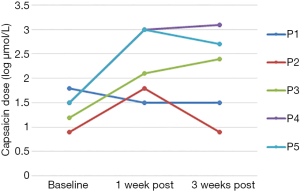
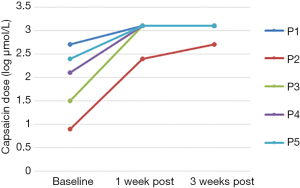
Wilcoxon’s Signed Rank Test, using the logC2 and logC5 values, revealed a significant difference relative to baseline, in logC5 at 1 week (z=−2.02, P=0.04) and 3 weeks (z=−2.02, P=0.04) post-treatment. The difference in logC2 neared significance at 1 week post-treatment (z=−1.77, P=0.077) but was insignificant at three weeks post-treatment (z=−1.46, P=0.144).
Discussion
This is the first study to investigate the potential of modifying cough reflex sensitivity through the use of a progressive desensitization model using increasing doses of aerosolized capsaicin paired with behavioral cough suppression. We were interested in testing this approach because of its potential to benefit patients who cannot suppress their cough in the presence of uncontrolled environmental stimuli, presumably making them less responsive to BCST. We hypothesize that such patients would benefit from BCST if they were able to successfully suppress their cough in the presence of a cough stimulant strong enough to elicit an UTC, but weak enough to allow for successful cough suppression. Given this is the first study to attempt such a treatment strategy, we chose to begin with healthy individuals to determine feasibility and proof-of-concept. Aerosolized capsaicin served as the cough stimulant given its established safety and reproducibility as well as the ability to dilute it to any concentration (39-42).
While this data is clearly very preliminary, and the sample size is extremely small, the results support our hypothesis. We demonstrated a change in cough threshold in five healthy individuals after just 2 weeks of desensitization treatment. While the change in C2 did not reach statistical significance, change in C5 was significant at both 1 and 3 weeks post-treatment, and was relatively stable in the post-treatment phase. Given that prior research suggests that C5 is a more reliable measure of cough sensitivity than C2, particularly for repeated testing (39), the data suggests these participants experienced a considerable change in cough sensitivity. Four of our five participants had such a large change they did not reach the C5 threshold at either post-treatment time points. Additionally, there were no reported or observed adverse effects from the treatment.
It may be questioned whether the change in C5 was due to participants simply becoming good at cough suppression rather than an actual change in cough sensitivity. Hutchins et al. [1993] (45) and Cho et al. [2017] (46) showed the ability of healthy participants to voluntarily suppress their cough during cough challenge testing by simply giving the instruction “please do not cough”. Given this evidence, it is reasonable to question whether our participants simply voluntarily suppressed their cough without using an overt cough suppression strategy. While this cannot be fully ruled out without a placebo or comparison group, we find it unlikely, given our explicit instruction to “not attempt to suppress the cough in any way.” Additionally, the C5 mean reported by Cho et al. during voluntary suppression trials in healthy individuals was 321.70 µmol/L, which is significantly lower than our data, of which only 1 of 5 participants even reached C5 at the highest dose of 1,000 µmol/L. Hutchins et al. only tested up to 333 µmol/L, and did not specifically measure C5, preventing a comparison across studies. Further, if the change in sensitivity testing were due solely to improvement in the ability to suppress the cough, it would not degrade the value of the treatment. Improved ability to easily suppress cough would help patients cope in social settings.
It may also be questioned whether the change in sensitivity was due solely to the repeated exposure to capsaicin, thereby negating the benefit of the added cough suppression. While marked tachyphylaxis has been demonstrated following repeated single-inhalation cough challenges (as was used in this study), existing data indicates that the attenuated response lasts no more than a few hours (47-49). This data is supported by studies showing reliable reproducibility of cough sensitivity testing with capsaicin at intervals of 20 minutes to 14 days (39,48-50). Given this information, we did not hypothesize that capsaicin exposure alone would cause desensitization and, therefore, did not include a no-suppression arm. Further, given the goals of the study was to determine feasibility and proof-of-concept, we felt little was to be gained by adding a no-suppression arm to testing on healthy volunteers. Future research comparing capsaicin exposure alone to capsaicin plus cough suppression in patients with RCC is needed to definitively answer this question. Still, if a desensitization effect is found to be due solely to repeated exposure to capsaicin, it will not necessarily negate the benefit of cough suppression, as being able to suppress the cough may increase tolerability of the treatment. In conclusion, we acknowledge several limitations to this study. The foremost being a very small sample size, lack of a placebo or comparison group to help elucidate the actual cause of the change in sensitization, and lack of inclusion of a clinical population. We also do not know the longevity of the effects beyond 3 weeks post-treatment. Given the novelty of the treatment concept, we thought it prudent to first demonstrate feasibility and proof-of-concept on healthy volunteers before testing on patients. With these goals now met, testing on patients with RCC using a randomized placebo-controlled design is appropriate.
If proven effective, this treatment model could be helpful for patients with RCC who do not respond well to BCST and have limited medical treatment options. Pharmaceutical options, such as neuromodulators, have been shown to be effective for up to 75% of patients (51); however, these medications have significant side effects, are costly, and not all patients experience long-lasting effects (51-53). Given the well-established safety of capsaicin exposure, and lack of adverse effects in our sample, the treatment posed in this study would likely have no remarkable contraindications or side effects. Further, considering the change participants in this study experienced after just 2 weeks of treatment, it may also prove to be a faster method of treatment than traditional BCST.
Acknowledgements
We would like to acknowledge the contribution of Dr. Sarjubhai Patel, PhD. who assisted with preparing the capsaicin solutions, and Serena Haller and Lyndsay Hutton, who assisted with data collection.
Funding: Funding for this study was provided in part by the Mountain West Clinical Translational Research Infrastructure Network under a grant from the National Institute of General Medical Sciences (No. 93.859) as well as from The University of Montana Small Grant Program (No. 325454).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by The University of Montana Institutional Review Board (#249-17) on November 28, 2017 for testing on healthy individuals. Written informed consent was obtained from each participant.
References
- Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet 2008;371:1364-74. [Crossref] [PubMed]
- Ford AC, Forman D, Moayyedi P, et al. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax 2006;61:975-9. [Crossref] [PubMed]
- Irwin RS, Boulet LP, Cloutier MM, et al. Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest 1998;114:133S-81S. [Crossref] [PubMed]
- Natt RS, Earis JE, Swift AC. Chronic cough: a multidisciplinary approach. J Laryngol Otol 2012;126:441-4. [Crossref] [PubMed]
- Polverino M, Polverino F, Fasolino M, et al. Anatomy and neuro-pathophysiology of the cough reflex arc. Multidiscip Respir Med 2012;7:5. [Crossref] [PubMed]
- Morice A. Chronic cough: epidemiology. Chron Respir Dis 2008;5:43-7. [Crossref] [PubMed]
- Brignall K, Jayaraman B, Birring SS. Quality of life and psychosocial aspects of cough. Lung 2008;186 Suppl 1:S55-8. [Crossref] [PubMed]
- French CL, Irwin RS, Curley FJ, et al. Impact of chronic cough on quality of life. Arch Intern Med 1998;158:1657-61. [Crossref] [PubMed]
- Young EC, Smith JA. Quality of life in patients with chronic cough. Ther Adv Respir Dis 2010;4:49-55. [Crossref] [PubMed]
- Slovarp L, Loomis BK, Glaspey A. Assessing referral and practice patterns of patients with chronic cough referred for behavioral cough suppression therapy. Chron Respir Dis 2018;15:296-305. [Crossref] [PubMed]
- Chung KF. Chronic 'cough hypersensitivity syndrome': a more precise label for chronic cough. Pulm Pharmacol Ther 2011;24:267-71. [Crossref] [PubMed]
- Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest 2005;127:1710-3. [Crossref] [PubMed]
- McGarvey LP, Heaney LG, Lawson JT, et al. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax 1998;53:738-43. [Crossref] [PubMed]
- Poe RH, Harder RV, Israel RH, et al. Chronic persistent cough experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest 1989;95:723-8. [Crossref] [PubMed]
- Chung KF. Approach to chronic cough: the neuropathic basis for cough hypersensitivity syndrome. J Thorac Dis 2014;6:S699-707. [PubMed]
- Chung KF, McGarvey L, Mazzone S. Chronic cough and cough hypersensitivity syndrome. Lancet Respir Med 2016;4:934-5. [Crossref] [PubMed]
- Francis DO, Slaughter JC, Ates F, et al. Airway Hypersensitivity, Reflux, and Phonation Contribute to Chronic Cough. Clin Gastroenterol Hepatol 2016;14:378-84. [Crossref] [PubMed]
- Morice AH. The cough hypersensitivity syndrome: a novel paradigm for understanding cough. Lung 2010;188 Suppl 1:S87-90. [Crossref] [PubMed]
- Groneberg DA, Niimi A, Dinh QT, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med 2004;170:1276-80. [Crossref] [PubMed]
- Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 2001;125:47-65. [Crossref] [PubMed]
- Ando A, Smallwood D, McMahon M, et al. Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax 2016;71:323-9. [Crossref] [PubMed]
- Vertigan AE, Gibson PG. Chronic refractory cough as a sensory neuropathy: evidence from a reinterpretation of cough triggers. J Voice 2011;25:596-601. [Crossref] [PubMed]
- Morice AH, Faruqi S, Wright CE, et al. Cough hypersensitivity syndrome: a distinct clinical entity. Lung 2011;189:73-9. [Crossref] [PubMed]
- Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2012;9:CD010111. [PubMed]
- Malek N, Pajak A, Kolosowska N, et al. The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol Cell Neurosci 2015;65:1-10. [Crossref] [PubMed]
- Moon JY, Lee PB, Kim YC, et al. Efficacy and Safety of 0.625% and 1.25% Capsaicin Patch in Peripheral Neuropathic Pain: Multi-Center, Randomized, and Semi-Double Blind Controlled Study. Pain Physician 2017;20:27-35. [PubMed]
- Ryan NM, Vertigan AE, Bone S, et al. Cough reflex sensitivity improves with speech language pathology management of refractory chronic cough. Cough 2010;6:5. [Crossref] [PubMed]
- Vertigan AE, Theodoros DG, Gibson PG, et al. Efficacy of speech pathology management for chronic cough: a randomised placebo controlled trial of treatment efficacy. Thorax 2006;61:1065-9. [Crossref] [PubMed]
- Chamberlain S, Garrod R, Birring SS. Cough suppression therapy: does it work? Pulm Pharmacol Ther 2013;26:524-7. [Crossref] [PubMed]
- Gibson PG, Vertigan AE. Speech pathology for chronic cough: a new approach. Pulm Pharmacol Ther 2009;22:159-62. [Crossref] [PubMed]
- Chamberlain Mitchell SA, Garrod R, Clark L, et al. Physiotherapy, and speech and language therapy intervention for patients with refractory chronic cough: a multicentre randomised control trial. Thorax 2017;72:129-36. [Crossref] [PubMed]
- Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and Speech Pathology Combination Therapy for Refractory Chronic Cough: A Randomized Controlled Trial. Chest 2016;149:639-48. [Crossref] [PubMed]
- Soni RS, Ebersole B, Jamal N. Treatment of Chronic Cough: Single Institution Experience Utilizing Behavioral Therapy. Otolaryngol Head Neck Surg 2017;156:103-8. [Crossref] [PubMed]
- Ryan NM, Vertigan AE, Gibson PG. Chronic cough and laryngeal dysfunction improve with specific treatment of cough and paradoxical vocal fold movement. Cough 2009;5:4. [Crossref] [PubMed]
- Patel AS, Watkin G, Willig B, et al. Improvement in health status following cough-suppression physiotherapy for patients with chronic cough. Chron Respir Dis 2011;8:253-8. [Crossref] [PubMed]
- Vertigan AE, Theodoros DG, Gibson PG, et al. Review series: chronic cough: behaviour modification therapies for chronic cough. Chron Respir Dis 2007;4:89-97. [Crossref] [PubMed]
- Murry T, Branski RC, Yu K, et al. Laryngeal sensory deficits in patients with chronic cough and paradoxical vocal fold movement disorder. Laryngoscope 2010;120:1576-81. [Crossref] [PubMed]
- Vertigan AE, Bone SL, Gibson PG. Development and validation of the Newcastle laryngeal hypersensitivity questionnaire. Cough 2014;10:1. [Crossref] [PubMed]
- Dicpinigaitis PV. Short- and long-term reproducibility of capsaicin cough challenge testing. Pulm Pharmacol Ther 2003;16:61-5. [Crossref] [PubMed]
- Brooks SM, Truncale T, Sams A. Laboratory safety of capsaicin inhalation in healthy younger and older populations potential template for inhalation research. J Allergy Ther 2016;7:3-8. [Crossref]
- Dicpinigaitis PV, Alva RV. Safety of capsaicin cough challenge testing. Chest 2005;128:196-202. [Crossref] [PubMed]
- Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J 2007;29:1256-76. [Crossref] [PubMed]
- Costanzo MT, Yost RA, Davenport PW. Standardized method for solubility and storage of capsaicin-based solutions for cough induction. Cough 2014;10:6. [Crossref] [PubMed]
- Vertigan AE, Gibson P. Speech Pathology Management of Chronic Refractory Cough and Related Disorders. UK: Compton Publishing, 2016.
- Hutchings HA, Morris S, Eccles R, et al. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir Med 1993;87:379-82. [Crossref] [PubMed]
- Cho PS, Fletcher H, Turner RD, et al. S32 Cough Suppression Test: A Novel Objective Test for Chronic Cough. Thorax 2017;72:A22-3.
- Morice AH, Higgins KS, Yeo WW. Adaptation of cough reflex with different types of stimulation. Eur Respir J 1992;5:841-7. [PubMed]
- Collier JG, Fuller RW. Capsaicin inhalation in man and the effects of sodium cromoglycate. Br J Pharmacol 1984;81:113-7. [Crossref] [PubMed]
- Fuller RW. Pharmacology of inhaled capsaicin in humans. Respir Med 1991;85:31-4. [Crossref] [PubMed]
- Ternesten-Hasséus E, Johansson EL, Millqvist E. Cough reduction using capsaicin. Respir Med 2015;109:27-37. [Crossref] [PubMed]
- Cohen SM, Misono S. Use of specific neuromodulators in the treatment of chronic, idiopathic cough: a systematic review. Otolaryngol Head Neck Surg 2013;148:374-82. [Crossref] [PubMed]
- Wei W, Liu R. The efficacy of specific neuromodulators on human refractory chronic cough: a systematic review and meta-analysis. J Thorac Dis 2016;8:2942-51. [Crossref] [PubMed]
- Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:1583-9. [Crossref] [PubMed]