
Behavioral defects induced by chronic social defeat stress are protected by Momordica charantia polysaccharides via attenuation of JNK3/PI3K/AKT neuroinflammatory pathway
Introduction
Depression is characterized by change in mood, such as feeling guilty, low self-worth, low energy and loss of interest or pleasure. Depression has been recognized as a globally prevalent psychiatric disorder according to the World Health Organization (1) owing to resistance to treatment and high risk of suicide. The commonly prescribed medicines have certain drawbacks, including a low curative ratio, limited spectrum of activity, slow onset of action and excessive side effects (2). Therefore, it is necessary to explore new antidepressants, which enable clinicians to diversify their treatment options.
Multiple clinical studies have shown that the mean levels of proinflammatory cytokines are elevated in the blood of depressed individuals. Many pro-inflammatory factors, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β), play an important role in the pathophysiological process of depression (3,4). In animal studies, researchers reported that depressed animals exhibit higher levels of pro-inflammatory cytokines (5) which might inhibit neurotransmission and plasticity, and suppress neurogenesis (6). Therefore, inflammation might be an important biological mechanism for depression. There are several signaling pathways involved in neurodegenerative pathology and mood diseases related to inflammation, such as the C-Jun N-terminal protein kinase (JNK) pathway.
JNK, a member of the mitogen-activated protein (MAP) kinases, consists of at least 10 JNK isoforms, which are encoded by three genes: Jnk1, Jnk2, Jnk3 (7). Altered distribution and activation of JNK3 in post-mortem brain sections suggests that JNK3 is involved in Alzheimer’s disease (8,9). Furthermore, stress-induced activation of JNK in the hippocampus is associated with emotional memories deficits, which can be improved by the administration of JNK inhibitors (10,11). Additionally, treatments with JNK inhibitors prevent the impairment of conditioned fear, indicating that JNK activity plays an important role in stress-impaired fear conditioning (12). Signal stressors delay the loss of apical dendrites and increase the number of basal dendritic trees (13), which elevates phosphorylation and activity of JNK in hippocampal sub-regions (12). JNK3 is abundantly expressed in the brain and functions as an essential player in the neuronal stress response in various neuropathological diseases, however, the underlying mechanisms differentially regulating JNK3 in depression remain unclear (14,15).
As a downstream target of JNK3, the protein p110 beta (P-110 β) is involved in the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway. The activity of PI3K and phosphorylation of AKT is higher in the hippocampus of Jnk3 mull mice (16). Evidence suggests that the PI3K/AKT pathway is involved in the pathophysiology of depression. Some antidepressant-like compounds target this pathway as well, which is involved in synaptic neurotransmission, neuronal cell proliferation, migration and plasticity (17-19). Specifically, AKT2 plays an important role in the process of neuronal differentiation, survival, and dopamine transporter cell surface expression (20,21). Furthermore, studies show that deficiency of AKT2 is associated with depressive-like behaviors in mice (22).
Momordica charantia (MC) is a popular vegetable and is consumed as food-medicine in traditional Chinese medicine. The polysaccharide of MC is one of the major active ingredients and contributors to the beneficial effects of MC (23,24). Studies suggest that Momordica charantia polysaccharide (MCP) possesses various beneficial effects, including antioxidant and anti-inflammatory responses, which are involved in the pathophysiology of depression (25,26). However, whether MCP contributes to neuroprotective effects against depressive-like behaviors induced by chronic social stress is still unknown. We hypothesize that MCP protects against depressive-like behaviors and decreases the expression of proinflammatory cytokines through the JNK3/PI3K/AKT signaling pathway in the chronic social defeat stress (CSDS) depressed animal model.
Methods
Animals
The 8-week-old male C57 mice (Experimental Animal Center of China Three Gorges University) were 8–10 weeks of age, the room temperature was 24±1 °C, and relative humidity was 55%±10% with 12-h light/dark cycle. After 1 week, in compliance with the National Institute of Health and the Animal Care, Inspection by members in The Medical Animal Care & Welfare Committee of Three Gorges University, the behavioral experiments were performed.
Drug preparation and administration
The preparation of MCP was described as previous publication (26). The MC was purchased from a local vegetable market in Yichang City of Hubei Province. Briefly, water extraction and alcohol precipitation were used to extract MCP from M. charantia. MCP was dissolved in distilled water.
LY294002 (Sigma Chemical, St Louis, MO, USA), a phosphatidylinositol-3-kinase (PI3-K) inhibitor, was administered via direct intraperitoneal injection (i.p.) 30 min after stress. Animals received LY294002 at a dose of 7.5 mg/kg, dissolved in dimethyl sulfoxide (DMSO) to a volume of 10 µL per gram of body weight.
Experimental design
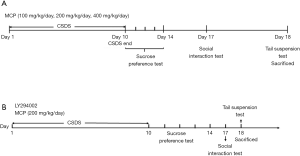
The CSDS mice model was established based on our previous study (27). The schematic timeline of the experimental procedures was shown in Figure 1. After establishment of CSDS model, the behavioral tests were performed.
Social interaction test (SIT)
A two-trial SIT was described previously (28). Briefly, there are two trials (“target absent” trial, “target present” trial) in this test, the time was last for 5 minutes in every trial. In the first trial, the behaviors of experimental C57 mouse were free in a 44 cm × 44 cm square-shaped open-field arena, which possessed a 10 cm × 6 cm wire-mesh cage opposed to one side. In the second trial, the target (an unfamiliar CD1 retired breeder mouse) was reintroduced into this arena. During the 5 minutes, the interaction time between the test C57 mouse and CD1 mouse in the interaction zone was recorded.
Sucrose preference test (SPT)
The SPT was carried out in 4 days. At first, pure water and 1% sucrose solution were supplied to the test mouse in 2 days, respectively. Then, the food and two bottles were both deprived in next 18 hours. The last day, the test was performed. The weights of two bottles were measured before and after the test.
Tail suspension test (TST)
This test was performed according to previous study (29). C57 mouse was individually suspended by adhesive tap (1 cm from the tail tip) 60 cm above the floor. The total test time was 6 minutes. The mouse was considered immobile only when it was hanging passively and completely motionless. The immobility time was recorded with a video camera. The recorder started to record the time and ended when the mouse mice to mobile. At the end of the test, the immobility time was accumulated. The observer who performed the TST test was unaware of animal grouping and treated each mouse equally.
PI3K activity assay
The bilateral hippocampus of sacrificed test mice was dissected. The hippocampus proteins were collected in NP-40 lysis buffer (Servicebio, Wuhan, China). After the lysis centrifuged (12,000 ×g, 15 minutes, 4 °C), collecting the supernatants. According to the manufacturer’s guidelines, PI3 Kinase assay kit (Echelon Biosciences, USA, catalog number: K-1000s) was used to determined PI3K activity.
Determination of proinflammatory cytokines
Commercially-available kits (R&D System, USA) were used to assay the concentrations of TNF-α (R&D System, USA, catalog number: MTA00B), IL-6 (R&D System, USA, catalog number: M6000B) and IL-1β (R&D System, USA, catalog number: MLB00C), which were expressed as pg/mL protein. The protein levels of tissue supernatant were estimated.
RT-PCR
The bilateral hippocampus of sacrificed test mice was dissected. The hippocampus total RNA was collected by isolated with the use of Trizol reagent (Invitrogen, San Diego, CA, USA). Then, total RNA (1 mg) was reverse transcribed, and the resulting cDNA (1 mL) was used to detect the transcripts. The primers used for pik3cb and GAPDH (Invitrogen) were:
pik3cb: forward 5'-CTATGGCAGACAACCTTGACAT-3', reverse 5'-CTTCCCGAGGTACTTCCAACT-3',
GAPDH: forward 5'-ACATTGTTG CCATCA ACGAC-3', reverse 5'-ACGCCAGTAGACTCCACGAC-3'.
Western blot
The bilateral hippocampus of sacrificed test mice was dissected. The hippocampus proteins were collected in RIPA buffer (Servicebio). After the lysis centrifuged (12,000 ×g, 15 minutes, 4 °C), collecting the supernatants. The quantified samples were loaded and separated by 8–12% SDS-PAGE gels and then transferred to PVDF membrane (0.45 µL, Millipore, USA). Membranes were blocked with 5% non-fat milk in TBST at room temperature for 1 h and then incubated with P110 β (Cell Signaling Technology, USA, catalog: 3011), JNK3 (Cell Signaling Technology, USA, catalog: 2305), c-Jun (Cell Signaling Technology, USA, catalog: 9165), phospho-AKT (Ser473) (Cell Signaling Technology, USA, catalog: 4060), total AKT (Cell Signaling Technology, USA, catalog: 4691), and β-actin (Cell Signaling Technology, USA, catalog: 3700) at 4 °C overnight. The membranes were incubated with a horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (1:3,000, Servicebio) at room temperature for 2 h. Bands were visualized with enhanced chemiluminescence (Clinx Science Instruments Co. China).
Statistical analysis
The data were showed as means ± SEM. The analysis was performed by the SPSS 13.0 software. The comparisons between groups were performed using one-way analysis of variance (ANOVA) followed by post hoc LSD test. P<0.05 was considered as statistically significant.
Results
MCP prevents depressive-like behaviors caused by CSDS
We examined the protective effects of MCP in the CSDS mouse model. The schematic timeline of the experimental procedures is shown in Figure 1. MCP was administered daily by gavage (at doses of 100, 200 and 400 mg/kg) 30 minutes before stress was induced. The behavioral tests suggested that there was a decrease in the interaction time of CSDS mice in SIT, and an increase in the immobility time in TST, compared to control mice. Furthermore, there was a reduction in sucrose preference in SPT (Figure 2). However, CSDS mice administered 200 and 400 mg/kg/day of MCP exhibited obvious improvements in their behavioral indexes.

MCP decreases the levels of proinflammatory cytokines
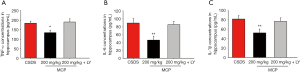
To explore the protective mechanisms of MCP, the levels of TNF-α, IL-6, and IL-1β in the hippocampus were analyzed. The levels of these pro-inflammatory cytokines were significantly higher in CSDS mice compared to the control group. However, administration of MCP decreased the levels of TNF-α, IL-6, and IL-1β in the hippocampus of CSDS mice (Figure 3).

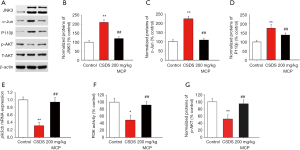
MCP inhibits JNK3 and c-Jun expression in the hippocampus of CSDS mice
The JNK pathway is a critical cellular signaling pathway and a target of anti-inflammation therapies for neuroprotection (29). Studies show that MCP significantly suppresses the activation of JNK3 in ischemia-reperfused brains (30). Protein expression levels of JNK3/c-Jun and AKT were analyzed using western blot. PI3K activity was also assayed (Figure 4). It was observed that the expression of JNK3, c-Jun and p110 β proteins were higher in CSDS mice than in control mice. However, the activity of PI3K, mRNA expression of pik3cb, and protein expression of phosphorylated AKT had decreased. Administration of MCP at a concentration of 200 mg/kg/day partially reversed the above observations.
MCP protects against the depressive-like behaviors of CSDS mice through the JNK3/PI3K/AKT pathway
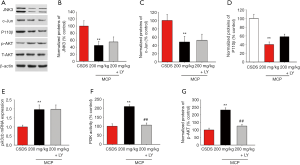
We observed that LY294002 (a PI3K inhibitor) partly abolished the antidepressant-like effects of MCP (Figure 5). The protective effects of MCP on SPT, SIT and TST were partly reversed by LY294002. The decrease in TNF-α, IL-6, and IL-1β expression levels in the hippocampus of CSDS mice after MCP administration were inhibited by LY294002 (Figure 6). Furthermore, compared to administration of MCP, the administration of LY294002 reduced the protein expression levels of phosphorylated AKT, inhibited PI3K activity and mRNA expression of pik3cb, and elevated the protein expression levels of JNK3, c-Jun and P110 β (Figure 7).

Discussion
The present study provides behavioral and neurochemical evidence to demonstrate that MCP has neuroprotective effects against depressive-like behaviors. Our results show that depressive-like behaviors induced by CSDS, as evaluated using SIT, SPT and TST, are similar to those of previous findings (31,32). More importantly, MCP significantly protects against these behavioral deficits, and inhibits the protein expression of JNK3 in the hippocampus of CSDS mice. This data suggests that a JNK3/PI3K/AKT cascade might be responsible for the effects of MCP in CSDS mice. MCP exhibits various interesting pharmacological properties such as inhibition of oxidative stress, inflammation and apoptosis (33). Previous studies also show that elevated levels of TNF-α, IL-6 and IL-1β are related to resistance and severity of depressive symptoms (34). In addition, depression is always associated with release of pro-inflammatory factors and an increase in neuronal cell death in the hippocampus (26). The present study demonstrates that TNF-α, IL-6, and IL-1β are up-regulated in the hippocampus of CSDS mice, and down-regulated after MCP treatment. These results strongly suggest that MCP has anti-depressant and anti-inflammatory effects. Thus, we hypothesize that MCP reduces inflammation and thereby decreases depressive-like behaviors.
JNK isoforms display distinct expression patterns in the hippocampus. JNK3 is observed in hippocampal sub-regions, as well as the dentate gyrus. Studies show that acute stress increases phosphorylation and activity of JNK within hippocampal sub-regions, and JNK inhibitors completely prevent the impairment of conditioned fear. These studies indicate that JNK activity is required for stress-impaired fear conditioning (12). JNK3 may be a crucial factor for stress-induced amnesia and might be important for neuroprotective therapy. A previous study suggests that there is a cross-talk between the JNK and PI3K pathway, specifically involving the JNK3 isoform. The study also shows that a lack of Jnk3 expression increases pik3cb transcript levels, which leads to an increase in PI3K activity (16). In this study, CSDS increased JNK3 expression and reduced pik3cb transcript levels. It also inhibited PI3K activity and decreased phosphorylated AKT expression. To the best of our knowledge, this study provides novel evidence linking the JNK3/PI3K/AKT pathway to depressive-like behaviors in CSDS mice, which can be mitigated by MCP intervention.
Brain derived neurotrophic factor (BDNF) has been strongly associated with depression and hippocampal neurogenesis (35). The activation of PI3K up-regulates the expression of VGF and BDNF in the hippocampus (36). Numerous studies have reported that PI3K, the main component of the PI3K/AKT pathway, plays an important role in the production of BDNF (22,37,38). Furthermore, AKT reduces depressive-like and manic-like behaviors by inhibiting GSK3 (39,40). Studies have also demonstrated that the PI3K/AKT/FoxO3a pathway functions as a target for fluoxetine (39). Our results show that LY294002 partly abolishes the antidepressant-like effects of MCP and reduces the protein expression levels of phosphorylated AKT, inhibits the activity of PI3K and mRNA expression of pik3cb, and elevates the protein expression levels of JNK3, c-Jun and P110 β. We therefore speculate that the ameliorative effects of MCP on depressive-like behaviors might be partly attributed to the regulation of the JNK3/PI3K/AKT pathway.
In summary, our study provides new insights in to understanding the pathophysiology of depression and the pharmacological effects of MCP. The belief that “natural is better” has led to public interest in herbal medicines, and to the development of antidepressants like ginsenosides and St. John’s wort (40,41). Our results shed light on the potential of MCP in preventing depression.
Acknowledgements
Funding: We gratefully acknowledge the National Natural Science Foundation of China (No. 81470387) for the generous financial support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study is in compliance with the National Institute of Health and the Animal Care, Inspection by members in The Medical Animal Care & Welfare Committee of Three Gorges University (No. 2017060q).
References
- Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095-105. [Crossref] [PubMed]
- Coupland C, Hill T, Morriss R, et al. Antidepressant use and risk of adverse outcomes in people aged 20-64 years: cohort study using a primary care database. BMC Med 2018;16:36. [Crossref] [PubMed]
- Lopresti AL, Hood SD, Drummond PD. Multiple antidepressant potential modes of action of curcumin: a review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J Psychopharmacol 2012;26:1512-24. [Crossref] [PubMed]
- Rawdin BJ, Mellon SH, Dhabhar FS, et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun 2013;31:143-52. [Crossref] [PubMed]
- Sukoff Rizzo SJ, Neal SJ, Hughes ZA, et al. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiatry 2012;2:e199. [Crossref] [PubMed]
- Hwang J, Zheng LT, Ock J, et al. Inhibition of glial inflammatory activation and neurotoxicity by tricyclic antidepressants. Neuropharmacology 2008;55:826-34. [Crossref] [PubMed]
- Gupta S, Barrett T, Whitmarsh AJ, et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J 1996;15:2760-70. [Crossref] [PubMed]
- Qi DS, Tao JH, Zhang LQ, et al. Neuroprotection of Cilostazol against ischemia/reperfusion-induced cognitive deficits through inhibiting JNK3/caspase-3 by enhancing Akt1. Brain Res 2016;1653:67-74. [Crossref] [PubMed]
- Reinecke K, Herdegen T, Eminel S, et al. Knockout of c-Jun N-terminal kinases 1, 2 or 3 isoforms induces behavioural changes. Behav Brain Res 2013;245:88-95. [Crossref] [PubMed]
- Bennett BL, Sasaki DT, Murray BW, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A 2001;98:13681-6. [Crossref] [PubMed]
- Borsello T, Clarke P G, Hirt L, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med 2003;9:1180-6. [Crossref] [PubMed]
- Sherrin T, Blank T, Hippel C, et al. Hippocampal c-Jun-N-terminal kinases serve as negative regulators of associative learning. J Neurosci 2010;30:13348-61. [Crossref] [PubMed]
- Kole MH, Costoli T, Koolhaas JM, et al. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience 2004;125:337-47. [Crossref] [PubMed]
- Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): differences revealed by gene targeting. Bioessays 2006;28:923-34. [Crossref] [PubMed]
- Brecht S, Kirchhof R, Chromik A, et al. Specific pathophysiological functions of JNK isoforms in the brain. Eur J Neurosci 2005;21:363-77. [Crossref] [PubMed]
- Junyent F, de Lemos L, Verdaguer E, et al. Gene expression profile in JNK3 null mice: a novel specific activation of the PI3K/AKT pathway. J Neurochem 2011;117:244-52. [Crossref] [PubMed]
- Budni J, Lobato KR, Binfare RW, et al. Involvement of PI3K, GSK-3beta and PPARgamma in the antidepressant-like effect of folic acid in the forced swimming test in mice. J Psychopharmacol 2012;26:714-23. [Crossref] [PubMed]
- Shi HS, Zhu WL, Liu JF, et al. PI3K/Akt signaling pathway in the basolateral amygdala mediates the rapid antidepressant-like effects of trefoil factor 3. Neuropsychopharmacology 2012;37:2671-83. [Crossref] [PubMed]
- Numakawa T, Adachi N, Richards M, et al. Brain-derived neurotrophic factor and glucocorticoids: reciprocal influence on the central nervous system. Neuroscience 2013;239:157-72. [Crossref] [PubMed]
- Vojtek AB, Taylor J, Deruiter SL, et al. Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol Cell Biol 2003;23:4417-27. [Crossref] [PubMed]
- Li G, Anderson RE, Tomita H, et al. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J Neurosci 2007;27:203-11. [Crossref] [PubMed]
- Leibrock C, Ackermann TF, Hierlmeier M, et al. Akt2 deficiency is associated with anxiety and depressive behavior in mice. Cell Physiol Biochem 2013;32:766-77. [Crossref] [PubMed]
- Xu X, Shan B, Liao CH, et al. Anti-diabetic properties of Momordica charantia L. polysaccharide in alloxan-induced diabetic mice. Int J Biol Macromol 2015;81:538-43. [Crossref] [PubMed]
- Liu X, Chen T, Hu Y, et al. Catalytic synthesis and antioxidant activity of sulfated polysaccharide from Momordica charantia L. Biopolymers 2014;101:210-5. [Crossref] [PubMed]
- Black CN, Penninx BW, Bot M, et al. Oxidative stress, anti-oxidants and the cross-sectional and longitudinal association with depressive symptoms: results from the CARDIA study. Transl Psychiatry 2016;6:e743. [Crossref] [PubMed]
- Kopschina Feltes P, Doorduin J, Klein HC, et al. Anti-inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. J Psychopharmacol 2017;31:1149-65. [Crossref] [PubMed]
- Jiang B, Wang F, Yang S, et al. SKF83959 produces antidepressant effects in a chronic social defeat stress model of depression through BDNF-TrkB pathway. Int J Neuropsychopharmacol 2014;18. [Crossref] [PubMed]
- Varty GB, Cohen-Williams ME, Hunter JC. The antidepressant-like effects of neurokinin NK1 receptor antagonists in a gerbil tail suspension test. Behav Pharmacol 2003;14:87-95. [Crossref] [PubMed]
- Wang T, Gu J, Wu PF, et al. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic Biol Med 2009;47:229-40. [Crossref] [PubMed]
- Gong J, Sun F, Li Y, et al. Momordica charantia polysaccharides could protect against cerebral ischemia/reperfusion injury through inhibiting oxidative stress mediated c-Jun N-terminal kinase 3 signaling pathway. Neuropharmacology 2015;91:123-34. [Crossref] [PubMed]
- Donahue RJ, Muschamp JW, Russo SJ, et al. Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry 2014;76:550-8. [Crossref] [PubMed]
- Kabir ZD, Lee AS, Burgdorf CE, et al. Cacna1c in the Prefrontal Cortex Regulates Depression-Related Behaviors via REDD1. Neuropsychopharmacology 2017;42:2032-42. [Crossref] [PubMed]
- Raish M. Momordica charantia polysaccharides ameliorate oxidative stress, hyperlipidemia, inflammation, and apoptosis during myocardial infarction by inhibiting the NF-kappaB signaling pathway. Int J Biol Macromol 2017;97:544-51. [Crossref] [PubMed]
- Suarez EC, Lewis JG, Krishnan RR, et al. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology 2004;29:1119-28. [Crossref] [PubMed]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 2002;82:1367-75. [Crossref] [PubMed]
- Wang W, Lu Y, Xue Z, et al. Rapid-acting antidepressant-like effects of acetyl-l-carnitine mediated by PI3K/AKT/BDNF/VGF signaling pathway in mice. Neuroscience 2015;285:281-91. [Crossref] [PubMed]
- Cross DA, Alessi DR, Cohen P, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995;378:785-9. [Crossref] [PubMed]
- Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci 2011;4:16. [Crossref] [PubMed]
- Zeng B, Li Y, Niu B, et al. Involvement of PI3K/Akt/FoxO3a and PKA/CREB Signaling Pathways in the Protective Effect of Fluoxetine Against Corticosterone-Induced Cytotoxicity in PC12 Cells. J Mol Neurosci 2016;59:567-78. [Crossref] [PubMed]
- Maher AR, Hempel S, Apaydin E, et al. St. John's Wort for Major Depressive Disorder: A Systematic Review. Rand Health Q 2016;5:12. [PubMed]
- Jiang B, Xiong Z, Yang J, et al. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol 2012;166:1872-87. [Crossref] [PubMed]


