
Histone deacetylase 6 selective inhibitor ACY1215 inhibits cell proliferation and enhances the chemotherapeutic effect of 5-fluorouracil in HCT116 cells
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-associated death worldwide, with an estimated 1.4 million cases and 693,900 deaths occurring in 2012 (1). Although precancerous adenoma and early stage CRC are more and more being discovered and subsequently removed with the development of screening and treatment strategy, more than 50% of the patients with CRC are diagnosed with advanced even metastatic cancer. Chemotherapy is an important even only method for these patients, and 5-fluorouracil (5-Fu)-based chemotherapy is the key therapy (2,3). However, many CRC patients may develop resistance to 5-Fu during treatment, which is associated with a high rate of recurrence and poor survival (4). Increasing evidence has demonstrated that epigenetic alterations play a major role in drug resistance in various cancers, including CRC (5-13). This suggests that epigenetic agents can be used to overcome CRC drug resistance and increase the efficacy of conventional chemotherapeutic agents in the clinic.
Acetylation and deacetylation are important epigenetic modifications, and play important roles in tumorigenesis and chemoresistance (14). Deacetylation is mediated by histone deacetylases (HDACs). HDAC6 is a unique class IIb HDAC, in that it is a predominant cytoplasmic protein with two catalytic domains, and it has demonstrated an ability to promote tumor growth in many human cancers (15). We found in our previous study that knockdown of HDAC6 could suppress proliferation, migration and invasion in HCT116 cells (16). Selective inhibition of HDAC6 exerts anticancer activity by inducing apoptosis, cell cycle arrest, responsiveness to chemotherapy, inhibition of cell proliferation and migration, etc. (17). ACY1215, also known as Ricolinostat, is the first oral HDAC6 selective inhibitor, and has shown promising results in multiple myeloma (MM) in preclinical studies and clinical trials (18-20). However, its role on colon cancer growth and the chemotherapeutic effect of 5-Fu on colon cancer remain largely undefined. The present study investigated the preclinical anticancer activity of ACY1215 in HCT116 cells and explored the impact of ACY1215 on the chemotherapeutic effect of 5-Fu in HCT116 cells.
Methods
Cell culture
The human colon cancer HCT116 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in a DMEM medium (Life Technologies, CA, USA) containing 10% fetal bovine serum (FBS), 100 units of penicillin, and 100 mg/mL streptomycin. The cells were maintained in 5% CO2 culture incubator at 37 °C.
Reagents and Antibodies
ACY1215 was purchased from Selleck Chemicals (Houston, TX, USA). Anti-phospho-MEK [9154], anti-MEK [8727], anti-phospho-ERK [3192], anti-ERK [4695] and anti-Ac-α-Tubulin [5335] antibodies were purchased from Cell Signaling. Anti-Tubulin (SC-73242) antibody was purchased from Santa Cruz. All antibodies were used in 1:1,000 dilutions in 5% non-fat milk for western blot.
Cell viability assay
In total, 3,000 cells/well were plated in 96-well plates and cultured in 5% CO2 culture incubator at 37 °C. After 24 h, cells were treated with different concentration of ACY1215 with or without 5-Fu. Twenty-four hours later, the cell viability was detected with the CellTiter-Glo Luminescent Cell Viability Assay kit according to the manufacturer’s instructions (Promega).
Colony formation assays
HCT116 cells treated with different concentrations of ACY1215 with or without 5-Fu were plated in 6-well culture dishes at a density of 1,000 cells/well and allowed to grow undisturbed for 10 days. Cells were stained with 1% crystal violet and the colony numbers (defined as >50 cells/colony) were counted.
In vitro scratch assay
HCT116 cells treated with different concentrations of ACY1215 with or without 5-Fu were plated in 6-well culture dishes. The cell monolayer was scraped in a straight line with a P200 pipet tip. Photographs of the scratch were taken at 0 and 48 h. Gap width at 0 h was set to 1. Gap width analysis was performed with Image J software. Measurements were taken at multiple defined sites (>6) along the scratch. Each scratch was given an average of all measurements. Data are expressed as the average of 3 independent experiments.
Cell migration assay
5×104 HCT116 cells treated with ACY1215 with or without 5-Fu were plated in an 8-µm, 24-well plate chamber insert (Corning Life Sciences, catalog no. 3422) with 100 µL serum-free medium at the top of the insert and DMEM medium (Gibco) containing 10% FBS (500 µL) at the bottom of the insert. Cells were incubated for 24 h and fixed with 4% paraformaldehyde for 15 min. After washing with PBS, cells at the top of the insert were scraped with a cotton swab. Cells adhering to the bottom were stained with 0.5% crystal violet blue for 15 min and then washed with double-distilled H2O. The positively stained cells were counted in 8 random fields under the microscope, and the average value of 8 fields was expressed. All assays were performed in triplicate.
Apoptosis assay
For the detection of apoptosis, cells treated with different concentrations of ACY1215 with or without 5-Fu for 36 h were co-stained with Annexin-V-PE and 7-AAD (Annexin V-PE Apoptosis Detection Kit I, BD Bioscience) and analyzed by fluorescence activated cell sorting (FACS) according to the manufacturer’s instructions.
Western Blot assay
Cells treated with different concentrations of ACY1215 were harvested and washed once by using cold PBS. The cell pellets were lysed in EBC buffer (50 mM Tris pH 7.5, 120 mM NaCl, 0.5% NP40) supplemented with protease inhibitors (Roche) and phosphatase inhibitors (Calbiochem). The lysates were cleared by centrifugation, and the lysates were quantified using a Beckman Coulter DU-800 spectrophotometer (Beckman Coulter) using the Bio-Rad protein assay reagent (Bio-Rad Laboratories, CA). The lysates were eluted by boiling for 5 minutes in SDS loading buffer. Bound proteins were resolved by SDS-PAGE and immunoblotted with the indicated antibodies.
Statistical analysis
Data was analyzed by SPSS 23.0 software (Chicago, IL, USA). Student t test was used to evaluate the significance between the groups. Error bars representing standard deviation. A P<0.05 indicated significance.
Results
ACY1215 suppresses HCT116 cells proliferation, migration and invasion, and induces apoptosis
After treating HCT116 cells with DMSO, and 1, 5 and 10 µM of ACY1215, the average relative cell viabilities were 1, 0.79, 0.57 and 0.37, respectively (Figure 1A); average colony numbers were 154.7, 121.7, 101 and 73, respectively (Figure 1B,C); average wound closure rates were 62.4%, 50.6%, 36.4% and 28.3%, respectively (Figure 1D,E); average migrated cell numbers were 80.2, 60.3, 48.5 and 29.2, respectively (Figure 1F,G). The cell viability, colony formation, wound closure and migrated cell numbers were significantly reduced with the increase of the concentration of ACY1215 (P<0.05). After treating HCT116 cells with DMSO, and 1, 5 and 10 µM of ACY1215, the proportion of apoptosis cells were 7%, 8.8%, 10.9% and 13.2%, respectively (Figure 1H,I), which was increased in a dose-dependent manner (P<0.05). These results together revealed that ACY1215 suppresses proliferation, migration and invasion, and promotes apoptosis in HCT116 cells.
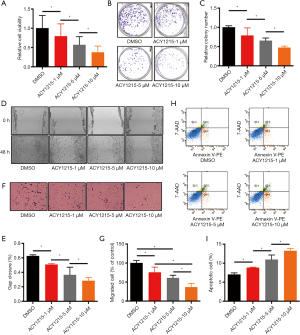
ACY1215 exerts its function partly through inhibition of MAPK/ERK signaling pathway
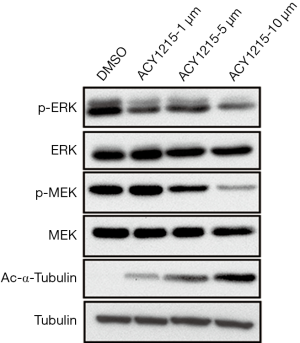
The MAPK/ERK pathway, also known as the Ras/Raf1/MEK/ERK pathway, is one of the most important signaling pathways involved in cell proliferation, migration and apoptosis (21). It has been demonstrated that activation of the MAPK/ERK pathway plays an important role in the pathogenesis, progression, and oncogenic behaviour of human CRC (22). Moreover, inhibitors targeting this pathway are a promising drug for treatment of CRC (23). As we have demonstrated that ACY1215 suppresses cell proliferation, migration and invasion, and promotes apoptosis, we further attempted to detect whether the function was due to inhibition of the MAPK/ERK pathway. After treating HCT116 cells with ACY1215, the expression of p-MEK and p-ERK decreased, while no obvious changes were noticed in the expression of MEK and ERK (Figure 2), suggesting that ACY1215 exerts its function partly through the inhibition of the MAPK/ERK pathway.
ACY1215 enhances the chemotherapeutic effect of 5-Fu in HCT116 cells
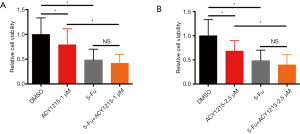
5-Fu is a major drug for chemotherapy of CRC, yet incomplete clinical response and/or chemoresistance are commonly observed, and combination therapy with other drugs could improve the chemotherapeutic effect, reduce chemo-resistance, and increase overall outcome (3,24). As ACY1215 exhibits anticancer activity in HCT116 cells, we therefore explored its impact on the chemotherapeutic effect of 5-Fu. As the results show, ACY1215 1 and 2.5 µM had no significant effect on the anticancer activity of 5-Fu in cell viability experiments (Figure S1A,B), while after treating HCT116 cells with DMSO, ACY1215 5 µM, 5-FU and ACY1215 5 µM + 5-FU, the average cell viabilities were 1, 0.57, 0.48 and 0.30, respectively (Figure 3A); average colony numbers were 121, 84, 68 and 41, respectively (Figure 3B,C); average wound closure rates were 59.7%, 40.4%, 38.6% and 22.1%, respectively (Figure 3D,E); average migrated cell numbers were 80.2, 48.5, 45.7 and 15.7, respectively (Figure 3F,G); the proportion of apoptosis cell were 7.9%, 13.4%, 17.5% and 24.4%, respectively (Figure 3H,I). The cell viability, colony formation, wound closure and migrated cell number were significantly reduced, and apoptotic cells increased when comparing control with single drug treatments, or comparing single drug with combined drug treatments (P<0.05). These results revealed that ACY1215 could synergistically enhance the anticancer activity of 5-Fu in HCT116 cells.
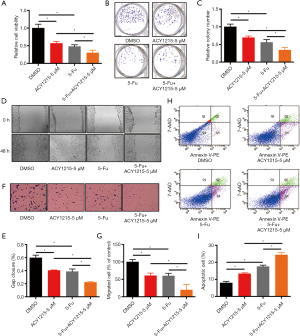
Discussion
In the present study, we found that ACY1215 could suppress HCT116 cell proliferation, migration and invasion, and induce apoptosis. Furthermore, ACY1215 synergistically enhances the anticancer activity of 5-Fu, as indicated by a decrease of cell proliferation, migration and invasion, and an increase in apoptotic cells. These findings suggested that targeting HDAC6 activity with ACY1215 may have presented a promising novel therapeutic strategy for colon cancer and enhance responsiveness of 5-Fu.
Histone deacetylase inhibitors (HDACIs) alter the acetylation status of histone and non-histone proteins to regulate various cellular events such as cell proliferation, differentiation, apoptosis, autophagy, angiogenesis and tumor immunity in tumor cells, and thus exhibit anticancer activity (25,26). HDACIs are a diverse group of compounds, which vary in structure, biological activity, and specificity, and can be subdivided into pan-HDACIs and selective HDACIs. At the present, four drugs, namely vorinostat (SAHA) (27), romidepsin (FK-228) (28), belinostat (PXD-101) (29) and panobinostat (LBH-589) (30) have granted the United States Food and Drug Administration’s approval for cancer treatment and several HDACIs are currently in various phases of clinical trials, either as mono-therapy and/or in combination with existing/novel anticancer agents (31). However, pan-HDACIs usually elicit profound side effects, such as haematological toxicity (thrombocytopenia and neutropenia), QT prolongation, digestive symptoms (fatigue, nausea, vomiting, diarrhea), because they target several HDAC isoforms, especially class I HDACs (32-34). Therefore, selective HDACIs that exhibit a more favorable side effect profile are imperative. It was reported that mice lacking HDAC6 develop normally with normal major organ function (35), implying that inhibition of HDAC6 may not cause major adverse events; thus, HDAC6 selective inhibitors are considered promising agents for cancer treatment.
At the present, several studies have reported the anticancer effect of HDAC6 selective inhibitors in colon cancer, and reported drugs included azaindolyl sulfonamides (36), aceroside VIII (37), 23bb (38), tubacin (39) and A452 (40,41). Each of these studies showed that HDAC6 selective inhibitors can suppress colon cancer cell growth, migration and invasion, and induce apoptosis, with the underlying mechanism involving p53, MAPK, DNA damage repair, etc. Unlike other HDAC6 selective inhibitors, ACY1215 is the only first-in class clinically relevant HDAC6 inhibitor and it has shown promising results in MM in preclinical study and clinical trials (18-20). Besides MM, ACY1215 has also been evaluated in clinical trials for treating breast cancer (NCT02632071), recurrent chronic lymphoid leukemia (NCT02787369), relapsed/refractory lymphoid malignancies (NCT02091063), and gynecological cancer (NCT02661815). However, the role it plays in colon cancer remains largely undefined. Recently, Lee et al. (42) found that ACY1215 could inhibit colon cancer growth and viability in a time- and dose-dependent manner. We also found that ACY1215 could inhibit HCT116 cell growth, viability, migration and invasion, and promote apoptosis in a dose-dependent way, which was in accordance with Lee’s study (42) and other studies concerning HDAC6 selective inhibitors in colon cancer (36-41). Also in accordance with previous studies (40-42), we found that ACY1215 exerted its anticancer effect partly through the MAPK/ERK signaling pathway, as indicated by a decreased expression of pMEK and pERK after cultured HCT116 cells with ACY1215, but no obvious change in MEK and ERK expression level.
Two studies have reported that HDAC6 selective inhibitors could enhance the anticancer effect of other anticancer agents in colon cancer. Lee et al. (42) found that ACY1215 could enhance the anticancer activity of oxaliplatin in CRC cells (HCT116 and HT29 cells), but they did not see a synergistic effect between ACY1215 and 5-Fu. Meanwhile, Won et al. (40) found that A452 could synergistically enhance the chemotherapeutic effect of irinotecan in CRC cells (HCT116 and HT29 cells). In the present study, we found that ACY1215 could enhance the anticancer effect of 5-Fu, as indicated by a decrease in cell proliferation, migration and invasion, and an increase in cell apoptosis. The difference between our results and Lee’s study (42) could be attributed to the use of a higher concentration of ACY1215 (5 µM) in our study, versus a lower one in Lee’s [2 µM in Lee’s study (42)]. The higher concentration is supposed to be more potent in suppressing tumor cell growth, in addition to providing a stronger effect on the anticancer activity of 5-Fu. Although inhibition of HDAC6 theoretically does not cause major adverse events, a higher concentration means more potential adverse events, thus further studies are warranted to explore the safety of ACY1215 and its optimal dose.
In conclusion, our study showed that the HDAC6 selective inhibitor ACY1215 could suppress cell proliferation, migration and invasion, and enhance the anticancer activity of 5-Fu in HCT116 cells, which provides a rationale for the combination of HDAC6 selective inhibitors with other anticancer agents in treating colon cancer.
Acknowledgements
Funding: The study was supported by the Chinese National Key Disciplines [no. (2012)650] and the Fundamental Research Funds from the Central Universities of Central South University (no. 2018zzts049).
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
References
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Lee JJ, Beumer JH, Chu E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother Pharmacol 2016;78:447-64. [Crossref] [PubMed]
- Benson AB 3rd, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:370-98. [Crossref] [PubMed]
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 2003;3:330-8. [Crossref] [PubMed]
- Crea F, Nobili S, Paolicchi E, et al. Epigenetics and chemoresistance in colorectal cancer: an opportunity for treatment tailoring and novel therapeutic strategies. Drug Resist Updat 2011;14:280-96. [Crossref] [PubMed]
- Ikehata M, Ogawa M, Yamada Y, et al. Different effects of epigenetic modifiers on the cytotoxicity induced by 5-fluorouracil, irinotecan or oxaliplatin in colon cancer cells. Biol Pharm Bull 2014;37:67-73. [Crossref] [PubMed]
- Okada K, Hakata S, Terashima J, et al. Combination of the histone deacetylase inhibitor depsipeptide and 5-fluorouracil upregulates major histocompatibility complex class II and p21 genes and activates caspase-3/7 in human colon cancer HCT-116 cells. Oncol Rep 2016;36:1875-85. [Crossref] [PubMed]
- Na YS, Kim SM, Jung KA, et al. Effects of the HDAC inhibitor CG2 in combination with irinotecan, 5-fluorouracil, or oxaliplatin on HCT116 colon cancer cells and xenografts. Oncol Rep 2010;24:1509-14. [PubMed]
- Arts J, King P, Mariën A, et al. JNJ-26481585, a novel "second-generation" oral histone deacetylase inhibitor, shows broad-spectrum preclinical antitumoral activity. Clin Cancer Res 2009;15:6841-51. [Crossref] [PubMed]
- Tumber A, Collins LS, Petersen KD, et al. The histone deacetylase inhibitor PXD101 synergises with 5-fluorouracil to inhibit colon cancer cell growth in vitro and in vivo. Cancer Chemother Pharmacol 2007;60:275-83. [Crossref] [PubMed]
- Fazzone W, Wilson PM, Labonte MJ, et al. Histone deacetylase inhibitors suppress thymidylate synthase gene expression and synergize with the fluoropyrimidines in colon cancer cells. Int J Cancer 2009;125:463-73. [Crossref] [PubMed]
- Xu DB, Wang YL, Yue Y, et al. Inhibitory effect of a novel histone deacetylases inhibitor FK228 on human colon cancer HCT-116 cells in vitro and in vivo. Zhonghua Zhong Liu Za Zhi 2013;35:814-8. [PubMed]
- Saito A, Yamashita T, Mariko Y, et al. A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc Natl Acad Sci U S A 1999;96:4592-7. [Crossref] [PubMed]
- Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007;26:5310-8. [Crossref] [PubMed]
- Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol 2011;2011:875824. [Crossref] [PubMed]
- Tan Y, Ci Y, Dai X, et al. Cullin 3SPOP ubiquitin E3 ligase promotes the poly-ubiquitination and degradation of HDAC6. Oncotarget 2017;8:47890-1. [Crossref] [PubMed]
- Yang PH, Zhang L, Zhang YJ, et al. HDAC6: physiological function and its selective inhibitors for cancer treatment. Drug Discov Ther 2013;7:233-42. [Crossref] [PubMed]
- Santo L, Hideshima T, Kung AL, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood 2012;119:2579-89. [Crossref] [PubMed]
- Yee AJ, Bensinger WI, Supko JG, et al. Ricolinostat plus lenalidomide, and dexamethasone in relapsed or refractory multiple myeloma: a multicentre phase 1b trial. Lancet Oncol 2016;17:1569-78. [Crossref] [PubMed]
- Vogl DT, Raje N, Jagannath S, et al. Ricolinostat, the First Selective Histone Deacetylase 6 Inhibitor, in Combination with Bortezomib and Dexamethasone for Relapsed or Refractory Multiple Myeloma. Clin Cancer Res 2017;23:3307-15. [Crossref] [PubMed]
- Sun Y, Liu WZ, Liu T, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 2015;35:600-4. [Crossref] [PubMed]
- Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol 2005;6:322-7. [Crossref] [PubMed]
- Martin-Liberal J, Lagares-Tena L, Larkin J. Prospects for MEK inhibitors for treating cancer. Expert Opin Drug Saf 2014;13:483-95. [Crossref] [PubMed]
- Temraz S, Mukherji D, Alameddine R, et al. Methods of overcoming treatment resistance in colorectal cancer. Crit Rev Oncol Hematol 2014;89:217-30. [Crossref] [PubMed]
- Manal M, Chandrasekar MJ, Gomathi Priya J, et al. Inhibitors of histone deacetylase as antitumor agents: A critical review. Bioorg Chem 2016;67:18-42. [Crossref] [PubMed]
- Lakshmaiah KC, Jacob LA, Aparna S, et al. Epigenetic therapy of cancer with histone deacetylase inhibitors. J Cancer Res Ther 2014;10:469-78. [PubMed]
- Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol 2007;25:3109-15. [Crossref] [PubMed]
- Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol 2009;27:5410-7. [Crossref] [PubMed]
- Foss F, Advani R, Duvic M, et al. A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol 2015;168:811-9. [Crossref] [PubMed]
- Richardson PG, Schlossman RL, Alsina M, et al. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood 2013;122:2331-7. [Crossref] [PubMed]
- Eckschlager T, Plch J, Stiborova M, et al. Histone Deacetylase Inhibitors as Anticancer Drugs. Int J Mol Sci 2017;18:E1414. [Crossref] [PubMed]
- Fraczek J, Vanhaecke T, Rogiers V. Toxicological and metabolic considerations for histone deacetylase inhibitors. Expert Opin Drug Metab Toxicol 2013;9:441-57. [Crossref] [PubMed]
- Zhang L, Han Y, Jiang Q, et al. Trend of histone deacetylase inhibitors in cancer therapy: isoform selectivity or multitargeted strategy. Med Res Rev 2015;35:63-84. [Crossref] [PubMed]
- Schiattarella GG, Sannino A, Toscano E, et al. Cardiovascular effects of histone deacetylase inhibitors epigenetic therapies: Systematic review of 62 studies and new hypotheses for future research. Int J Cardiol 2016;219:396-403. [Crossref] [PubMed]
- Zhang Y, Kwon S, Yamaguchi T, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol 2008;28:1688-701. [Crossref] [PubMed]
- Lee HY, Tsai AC, Chen MC, et al. Azaindolylsulfonamides, with a more selective inhibitory effect on histone deacetylase 6 activity, exhibit antitumor activity in colorectal cancer HCT116 cells. J Med Chem 2014;57:4009-22. [Crossref] [PubMed]
- Ryu HW, Lee DH, Shin DH, et al. Aceroside VIII is a new natural selective HDAC6 inhibitor that synergistically enhances the anticancer activity of HDAC inhibitor in HT29 cells. Planta Med 2015;81:222-7. [Crossref] [PubMed]
- Yang Z, Wang T, Wang F, et al. Discovery of Selective Histone Deacetylase 6 Inhibitors Using the Quinazoline as the Cap for the Treatment of Cancer. J Med Chem 2016;59:1455-70. [Crossref] [PubMed]
- Chao OS, Chang TC, Di Bella MA, et al. The HDAC6 Inhibitor Tubacin Induces Release of CD133+ Extracellular Vesicles From Cancer Cells. J Cell Biochem 2017;118:4414-24. [Crossref] [PubMed]
- Won HR, Ryu HW, Shin DH, et al. A452, an HDAC6-selective inhibitor, synergistically enhances the anticancer activity of chemotherapeutic agents in colorectal cancer cells. Mol Carcinog 2018;57:1383-95. [Crossref] [PubMed]
- Ryu HW, Shin DH, Lee DH, et al. A potent hydroxamic acid-based, small-molecule inhibitor A452 preferentially inhibits HDAC6 activity and induces cytotoxicity toward cancer cells irrespective of p53 status. Carcinogenesis 2018;39:72-83. [Crossref] [PubMed]
- Lee DH, Won HR, Ryu HW, et al. The HDAC6 inhibitor ACY1215 enhances the anticancer activity of oxaliplatin in colorectal cancer cells. Int J Oncol 2018;53:844-54. [PubMed]


