Regional distribution of transpulmonary pressure
Transpulmonary pressure (PL) and structural components of the alveolo-capillary membrane
During spontaneous breathing in normal subjects, the inspiratory effort that is exerted by respiratory muscles decreases the already negative pleural pressure further, and drags lung tissue from the resting position (functional residual capacity) toward a superior point in the pressure-volume (PV) curve. The pressure gradient across the lung is termed “transpulmonary pressure” (PL).
Positive pressure mechanical ventilation differs considerably from the physiologic negative pressure that is generated by spontaneous breathing, as present in humans. In normal ventilation, humans are able to vary the breathing pattern within specific amplitude and time domains (1), and increase/decrease ventilation rate as a consequence of metabolic fluctuations. On the other hand, mechanical ventilators pressurize the respiratory system using a tidal volume (VT), positive end-expiratory pressure (PEEP), respiratory rate (RR), and inspiratory airway flow (V’), which are adjusted by the operator. During spontaneous breathing or positive mechanical ventilation, for the overall lung expansion, certain components of the alveolar-capillary membrane need to be stretched by PL, for example epithelial and endothelial cells, fibroblasts and extracellular matrix (ECM) itself. Importantly, both the degree and the rate of stretch each of these cells as well as matrix components may promote damage, resulting in so-called ventilator-induced lung injury (VILI) (2).
Despite their relatively high numbers and importance in terms of responsiveness to cyclic stretch by genomic alterations (3), as well as synthesis and secretion of ECM proteins (4), the contribution of cells to the mechanical properties of lungs is comparatively small. In fact, collagen, elastin and proteoglycans (PGs) confer the majority of mechanical properties of the lungs. Collagen represents one of the major proteins resisting the load induced by large PL variation, while elastin confers the elastic recoil to the resting position (5). Other important load-bearing proteins are glycoproteins (fibronectin and laminin), glycosaminoglycans (GAGs), and PGs (6). The non-fibrilar component, mainly hyaluron, and PG, connects the collagen and elastin fibers extremities, which can stabilize the ECM scaffold (2). It has been acknowledged that there is a threshold where the stress-strain relationship of this compartment loses its linearity, which corresponds to the point at which the ECM fibers of the lung skeleton become fully unfolded.
PL is heterogeneously distributed across the vertical gradient of the lung
Since the introduction of radioactive gases in investigations of the respiratory system (7,8), it is well known that the regional distribution of inspired gas across normal lungs is heterogeneous, being partly explained by the elastic properties of the lungs and vertical gradient of pleural pressure (9). For example, at low lung volume (below the resting position, i.e. FRC), the most dependent lung zones can be exposed to more positive pleural pressure and, as a consequence, are the first to close their distal airways during deflation and the last to reopen them during the next inflation, which in turn can affect the static volume-pressure (PV) relationship of the lungs (10). The PV curve can also be affected by posture. Washko et al. showed in healthy subjects that the average decrease in PL from the upright to supine is lower than 3 cmH2O (11). Nevertheless, even adding 3 cmH2O for a compensation shift of the PV curve, healthy individuals may still show negative values of PL. The authors have used esophageal pressure (Pes) manometry as surrogate of pleural pressure variation, and it is clear that Pes, at best, reflects the pressure in neighbor pleural space regions, overestimating it in non-dependent regions due to mediastinum mass displacement, and underestimating it in dependent areas (12).
There are different methods that can be used to calculate PL, ranging from direct measurement of PL at end-expiration to the elastance derived method at end-inspiration. Also, transpulmonary driving pressure (ΔPL) can be calculated in different ways. All those methods show advantages and have limitations (13). Recently, it has been pointed out some implications about these different methods of PL calculation:
Direct measurement of PL
After placing intra-pleural sensors both in dependent and non-dependent regions in large animal model of lung injury and human cadavers, Yoshida et al. (14) were able to show that the esophageal pressure balloon, that is, the Pes measurement, more closely reflects the intra-pleural pressure in dependent lung zones. In addition, the measurements were done at different PEEP levels. At the lowest PEEP level, PL at end-expiration was negative for both animal and human cadaver models. If they were placed according to an esophageal-pressure-guided protocol (15), they would have their PEEP levels adjusted at 8 and 10 cmH2O, respectively, without taking into account the oxygenation index. In healthy individuals, it has been shown that the superimposed pressure across the vertical gradient is approximately 4.5 cmH2O (16). Since the Pes signal better reflects the pressure conditions around itself, it is conceivable that the PEEP level in subjects would be adjusted to 5 cmH2O (15). On the other hand, in ARDS patients, the most dependent lung region can achieve superimposed pressure as high as 10.5 cmH2O, which might represent the lowest value of PEEP in these patients when aiming to keep those zones open at end-expiration.
Elastance derived method at end-inspiration of PL
This is a simplified method that computes PL as the product of the airway pressure during tidal inflation and the ratio between lung and respiratory system elastance. Thereby, the measurement of absolute Pes is not required. On the other hand, one should assume that the lung and respiratory system PV curves are linear in the range of PEEP and VT used in clinical settings (17). Recently, Yoshida et al. (14) showed that the elastance derived method at end-inspiration overestimated PL. Authors concluded that this would not necessarily invalidate the method, but instead indicated that this technique delivered the highest level of inspiratory lung stress, which might be guide adjustments of mechanical ventilation aimed at avoiding VILI. Another study showed that the PEEP level necessary to reach an end-expiratory PL of 0 cmH2O differed from the PEEP level targeting a chest-wall elastance-based end-inspiratory PL of 26 cmH2O (18). Thus, in ARDS patients, a combination of both methods would be misleading.
Transpulmonary driving pressure (ΔPL)
The transpulmonary driving pressure (ΔPL) is defined as the difference between PL at end-inspiration and PL at end-expiration. It reflects the distending pressure taken by the lungs when VT is delivered. The use of ΔPL offers some advantages against absolute PL values. First, ΔPL does not account for the static stress caused by PEEP, which contributes less to lung injury and sometimes can mitigate it (19). Second, ΔPL is not influenced by the distending pressure necessary to move the chest wall (20). Third, it is conceivable the ΔPL would better reflect the presence of regional inhomogeneity in mechanical properties. An increase in ΔPL would better suggest local tissue tension than respiratory system parameters which may carry some artifacts (21). Hence, it seems that ΔPL might be a better surrogate of lung stress and may even be a better predictor of clinical outcomes than ΔP (22). ΔPL is calculated as:
ΔPL = (PPLAT – PESO, end-insp) – (PEEPTOT − PESO, end-exp) [1]
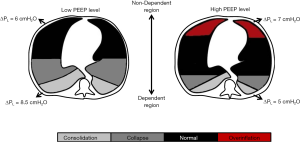
Further analyzing the Yoshida’s paper, about regional PL, it can be drawn some conclusions about regional ΔPL (Inspiration – Expiration) due to the presence of dependent and non-dependent sensors at different PEEP levels. At low PEEP level (4 cmH2O), in which the authors recognized the presence of ~30% atelectasis, the inspiratory and expiratory PL were 6 and −2.5 cmH2O, and the ΔPL was 8.5 cmH2O at dependent areas, higher than the non-dependent, in which the ΔPL was 6 cmH2O. Likely, the regional increment in ΔPL at dependent area can be dependent of opening and closing instability, in which some areas may suffer from cyclic overdistension. At a high PEEP level (for example, 24 cmH2O), in the absence of atelectasis, the ΔPL was 7 cmH2O in non-dependent areas, and 5 cmH2O in dependent areas, likely reflecting locally overdistension (Figure 1). Although these values seem relatively low, it must be kept in mind that they reflect the lung compartment only. Nevertheless, during assisted spontaneous breathing the effect of regional ΔPL can be very extreme.
PL during assisted spontaneous breathing: unraveling effector
A previous experimental study using pleural sensors showed a uniform distribution of ΔPL along the gravitation gradient during spontaneous breathing (23). However, this was assessed in healthy subjects, and probably may reflect the homogeneous stress distribution across the lung surface, by decreasing Ppl at all points. In contrast, in lung disease, the stress distribution may be inhomogeneous, mainly due to altered ventilation and the shape of diaphragm. Yoshida et al. showed that in an injured lung, local negative pleural pressure generated by diaphragmatic contraction is not uniformly transmitted, but is concentrated in dependent lung done. More importantly, esophageal manometry underestimated the respiratory efforts swings (24). It’s reasonable that, during lung-protective ventilation with low VT, the presence of increased inspiratory efforts can result in a hidden and local PL of the dependent lung. In this line, Bellani et al. compared the ΔPL during spontaneous assisted breathing and fully controlled ventilation, trying to match similar conditions of airflow and volume between both ventilatory conditions (25). For this purpose, in order to achieve matched volumes and airflows, the authors performed the analysis only on combinations of similar mean inspiratory flows between spontaneous assisted and controlled ventilations, discarding the others combinations of volumes and airflows for the same timeframe. Although, this reflects the careful methodologic approach performed by the authors, it poorly reflects the clinical condition presented by critical ill patient with distinct airflow and volumes. In addition, the effect of ΔPL whether in spontaneous assisted or controlled ventilation on gas exchange and global hemodynamics could not be evaluated. On the other hand, Henzler et al. (26) studied the effects of compared spontaneous breathing during BIPAP with controlled ventilation by matching ΔPL on gas exchange and hemodynamics. Interestingly, they showed similar effects on arterial partial oxygen pressure, but higher oxygen delivery in spontaneous breathing during BIPAP than controlled ventilation, which may be related to better hemodynamic. In order to achieve comparable ΔPL between spontaneous breathing and controlled ventilation, VT was increased up to 12 ml/kg, one more indicative that comparable ΔPL cannot be achieved by similar VT and airflow between spontaneous breathing and controlled ventilation.
High ΔPL may cause harm to the lungs but does the way that it is delivered matter?
During controlled ventilation, ΔPL is achieved by a positive increase in airway and pleural pressures, leading to compressive stress into the lung structure. Although, it’s widely accepted that alveolar pressure rarely shows lower values compared to pleural pressure, the delivery of stress into the alveolar surface during controlled ventilation (compressive stress) can substantially differ from that observed during spontaneous breathing (tensile stress). In fact, this has been observed at the cellular level (27). One way to compare compressive and tensile stresses is through the application of whole-body chamber and the application of negative pressure to the thorax and/or abdomen. In this line, Grasso et al. showed that ventilation with negative pressure may fundamentally differ from that achieved with positive pressure in terms of transmission of applied pressure, development of transpulmonary distending pressure, volumes of recruited lung, and distribution of lung aeration during inspiration and expiration. Additionally, both negative and positive pressures ventilation were matched by VT of 12 mL/kg, which nowadays, would not be feasible to clinical practice in ARDS condition. In a sequential study, by applying constant or transient negative abdominal pressures, authors showed that applying a distending pressure over a broad abdominal area associated to PEEP led to the recruitment of atelectatic areas, closer to diaphragmatic lung regions. In addition, the application of continuous negative abdominal pressure with PEEP or PEEP alone led to similar values of esophageal pressure. This reflects that regional recruitment did not affect overall chest wall compliance, and could be more related to a region juxta diaphragmatic. In fact, this has been observed elsewhere (24). More recently, continuous negative abdominal pressure (−5 cmH2O) was associated to protection against VILI (28). The mechanism of protection may rely on the selective recruitment of dorsal atelectatic lung, which may increase the area able to be ventilated and reduce the stress across caused by VT. This regional recruitment is achievable by mitigating the abdominal influence into the thoracic cavity and reducing Ppl in the dependent regions, which in turn can increase the regional PL. This regional recruitment is different from that obtained by PEEP increase, which may overinflate those regions already inflated, usually observed in non-recruitable lungs (29). Additionally, in rats, it has been shown that gentle spontaneous breathing with esophageal pressure variation around 2.5 cmH2O and moderate PEEP level reduced inflammatory mediators as also maintained epithelial integrity (30). The esophageal decay and the generated PL performed in rats represents one-half to that performed in healthy humans (31), reaching values around 5 cmH2O for a quite breathing pattern. Therefore, taking into account the magnitude of decrease in continuous negative abdominal pressure done in experimental scenario (−5 cmH2O), it is quite conceivable to have these beneficial effects of spontaneous breathing, which would represent the tensile stress, in mild ARDS patients with gentle inspiratory efforts (−5 cmH2O) (32) when medium levels of pressure support are adjusted (25).
Regional distribution of ΔPL and edema development: Can interstitium and lymph flow intermediate this interaction?
Increased inspiratory efforts during mechanical ventilation reduce intrapleural pressure which can achieve sub-atmospheric, i.e., negative levels. Pleural space has been acknowledged as an interstitial tissue devoid of solid matrix (33) and its pressure can be considered the sum of pleural liquid pressure (Pliq) with dynamic deformation pressure (Pdef) exerted at focal points of contacts between sliding surfaces. The lymphatic flux depends on lymphatic conductance and intraluminar hydraulic pressure (Plymph). It has been shown that Plymph significantly decreases during spontaneous breathing, but not in controlled mechanical ventilation. This depicts that to produce and move lymph fluids from peripheral regions of the lung forward, there is a dependence of cardiorespiratory activity (34,35). Interesting, it has been shown a dependence of lung height for fluids propagation from peripheral regions of lung parenchyma and regional pleural lymphatic flux toward drainage thoracic lymph tracts. From the highest (non-dependent) to the lowest (dependent) sites of pleural compartments, there is a progressively increase in lymphatic flux, supporting the existence of local differences in fluid dynamics in the pleural and lung compartments. The higher lymphatic flux at the dependent lung regions added by the intense ΔPL presented at this region may favor the occurrence of interstitial and/or alveolar edema formation. At the initial stage of edema formation, fluid accumulation in lung parenchyma is mainly prevented by lymphatic activity (36). However, mechanical ventilation with the presence of inspiratory effort is associated to local increment in ΔPL. These large swings may disturb ECM organization (27) and provoke distortion or decrement in PGs content, which have important roles in maintaining the dryness of lung tissue (35) by pushing outward against the nonextensible collagen fibers due to their energy of hydration (27). In addition, the increase in pulmonary capillary permeability, inherent to several lung conditions, can likely increase the translocation of fluids from interstitium toward alveolar space (37). Within this scenario, the lymph flow activity may not pace the rhythm of edema formation, which at last stage is characterized by flooded alveoli, mainly at the dependent lung regions.
Considering the preservation of lymph flow conductance, the maintenance of mild-to-moderate spontaneous breathing during mechanical ventilation may enhance lymph flow and contribute to clearance of edema from lungs (38). This beneficial effect was not observed after solely PEEP application (39). However, it is not clear whether this exchange of fluids from thoracic to abdominal cavities may be dangerous. Although the communication between abdominal and thoracic cavities through the lymph flow has been reported (40,41), the likely consequences of this dangerous talk are poorly described. Papazian et al. (42) showed that the early administration of a neuromuscular blocking agent improved the adjusted 90-day survival and increased the time off the ventilator without increasing muscle weakness. One of the attributed reasons for clinical improvement was the prevention of asynchrony and decrease in metabolic needs demanded by the respiratory muscles in the early phase of mechanical ventilation in ARDS (43). One contributing factor could be the dissemination of inflammatory cells and their milieu from the thoracic cavity where the lungs is situated toward abdominal cavity perpetuating the distal inflammatory response. This phenomenon has been observed in intestinal ischemia and reperfusion experimental studies causing bronchial hyper-reactivity (44,45). Whether the implementation of assisted spontaneous breathing during the early phase of ARDS, when the primary cause to institute the mechanical ventilation has not been solved, may perpetuate distal inflammation through lymph flow is not known. In a previous European cohort study, Esteban et al. (46) showed that the weaning period represents around 40% of the total duration of stay in ICU. Similar proportion could be admitted in a recent and large observational study about the prevalence of ARDS (47), since the total time of invasive mechanical ventilation was comparable (around 8 days). Experimental studies have highlighted that spontaneous breathing promotes trans-diaphragmatic lymphatic return (48), which may affect extrapulmonary organs. Taking into account the regional PL at the juxta-diaphragmatic area with strong efforts (24), it is possible that this region could represent the source of dissemination of inflammatory cells, large molecules and plasma contents able to trigger and perpetuate inflammation in distal organ (Figure 2).

Conclusions
The net increment in pressure domain related to the lung expansion represents the PL, which can distort some important components of the alveolar-capillary membrane. This distortion promoted by PL is heterogeneous across the vertical gradient of the lungs. There are different forms of calculation of PL, each one with own strengths and limitations. The heterogeneous distribution of PL seems to be important in several conditions during mechanical ventilation, but especially during assisted spontaneous breathing, where pleural pressure swings could be magnified at the dependent regions if inappropriate PEEP levels are used. The consequences of this localized PL are not fully recognized. Here, we pointed out the importance of this PL heterogeneity related to lymph flow drainage, which can communicate thoracic and abdominal cavities and contribute to spread inflammation.
Acknowledgements
Funding: This work was supported by grants from the Carlos Chagas Filho Rio de Janeiro State Research Foundation (FAPERJ) (grant number E-26/202.869/2015), Rio de Janeiro, Brazil; and the Brazilian Council for Scientific and Technological Development (CNPq) (grant number 471438/2012-0), Brasília, Brazil.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tobin MJ, Mador MJ, Guenther SM, et al. Variability of resting respiratory drive and timing in healthy subjects. J Appl Physiol (1985) 1988;65:309-17. [PubMed]
- Silva PL, Negrini D, Rocco PR. Mechanisms of ventilator-induced lung injury in healthy lungs. Best Pract Res Clin Anaesthesiol 2015;29:301-13. [Crossref] [PubMed]
- Yerrapureddy A, Tobias J, Margulies SS. Cyclic stretch magnitude and duration affect rat alveolar epithelial gene expression. Cell Physiol Biochem 2010;25:113-22. [Crossref] [PubMed]
- Roan E, Waters CM. What do we know about mechanical strain in lung alveoli? Am J Physiol Lung Cell Mol Physiol 2011;301:L625-35. [Crossref] [PubMed]
- Gattinoni L, Carlesso E, Cadringher P, et al. Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur Respir J Suppl 2003;47:15s-25s. [Crossref] [PubMed]
- Cruz FF, Rocco PRM, Pelosi P. Role of the extracellular matrix in the genesis of ventilator-induced lung injury. Med Klin Intensivmed Notfmed 2018;113:2-6. [Crossref] [PubMed]
- Ball WC Jr, Stewart PB, Newsham LG, et al. Regional pulmonary function studied with xenon 133. J Clin Invest 1962;41:519-31. [Crossref] [PubMed]
- Glaister DH. The effect of posture on the distribution of ventilation and blood flow in the normal lung. Clin Sci 1967;33:391-8. [PubMed]
- Lai-Fook SJ, Beck KC, Southorn PA. Pleural liquid pressure measured by micropipettes in rabbits. J Appl Physiol Respir Environ Exerc Physiol 1984;56:1633-9. [PubMed]
- Sutherland PW, Katsura T, Milic-Emili J. Previous volume history of the lung and regional distribution of gas. J Appl Physiol 1968;25:566-74. [Crossref] [PubMed]
- Washko GR, O'Donnell CR, Loring SH. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol (1985) 2006;100:753-8. [PubMed]
- Hubmayr RD, Walters BJ, Chevalier PA, et al. Topographical distribution of regional lung volume in anesthetized dogs. J Appl Physiol Respir Environ Exerc Physiol 1983;54:1048-56. [PubMed]
- Grieco DL, Chen L, Brochard L. Transpulmonary pressure: importance and limits. Ann Transl Med 2017;5:285. [Crossref] [PubMed]
- Yoshida T, Amato MBP, Grieco DL, et al. Esophageal Manometry and Regional Transpulmonary Pressure in Lung Injury. Am J Respir Crit Care Med 2018;197:1018-26. [Crossref] [PubMed]
- Talmor D, Sarge T, O'Donnell CR, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med 2006;34:1389-94. [Crossref] [PubMed]
- Pelosi P, D'Andrea L, Vitale G, et al. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;149:8-13. [Crossref] [PubMed]
- Chiumello D, Cressoni M, Colombo A, et al. The assessment of transpulmonary pressure in mechanically ventilated ARDS patients. Intensive Care Med 2014;40:1670-8. [Crossref] [PubMed]
- Gulati G, Novero A, Loring SH, et al. Pleural pressure and optimal positive end-expiratory pressure based on esophageal pressure versus chest wall elastance: incompatible results*. Crit Care Med 2013;41:1951-7. [Crossref] [PubMed]
- Protti A, Andreis DT, Monti M, et al. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med 2013;41:1046-55. [Crossref] [PubMed]
- Cortes-Puentes GA, Keenan JC, Adams AB, et al. Impact of Chest Wall Modifications and Lung Injury on the Correspondence Between Airway and Transpulmonary Driving Pressures. Crit Care Med 2015;43:e287-95. [Crossref] [PubMed]
- Marini JJ, Jaber S. Dynamic predictors of VILI risk: beyond the driving pressure. Intensive Care Med 2016;42:1597-600. [Crossref] [PubMed]
- Baedorf Kassis E, Loring SH, Talmor D. Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med 2016;42:1206-13. [Crossref] [PubMed]
- Minh VD, Friedman PJ, Kurihara N, et al. Ipsilateral transpulmonary pressures during unilateral electrophrenic respiration. J Appl Physiol 1974;37:505-9. [Crossref] [PubMed]
- Yoshida T, Torsani V, Gomes S, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 2013;188:1420-7. [Crossref] [PubMed]
- Bellani G, Grasselli G, Teggia-Droghi M, et al. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care 2016;20:142. [Crossref] [PubMed]
- Henzler D, Dembinski R, Bensberg R, et al. Ventilation with biphasic positive airway pressure in experimental lung injury. Influence of transpulmonary pressure on gas exchange and haemodynamics. Intensive Care Med 2004;30:935-43. [Crossref] [PubMed]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J 2006;20:811-27. [Crossref] [PubMed]
- Yoshida T, Engelberts D, Otulakowski G, et al. Continuous Negative Abdominal Pressure Reduces Ventilator-induced Lung Injury in a Porcine Model. Anesthesiology 2018;129:163-72. [Crossref] [PubMed]
- Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006;354:1775-86. [Crossref] [PubMed]
- Magalhaes PAF, Padilha GA, Moraes L, et al. Effects of pressure support ventilation on ventilator-induced lung injury in mild acute respiratory distress syndrome depend on level of positive end-expiratory pressure: A randomised animal study. Eur J Anaesthesiol 2018;35:298-306. [PubMed]
- Thammanomai A, Hamakawa H, Bartolak-Suki E, et al. Combined effects of ventilation mode and positive end-expiratory pressure on mechanics, gas exchange and the epithelium in mice with acute lung injury. PLoS One 2013;8. [Crossref] [PubMed]
- Yoshida T, Uchiyama A, Fujino Y. The role of spontaneous effort during mechanical ventilation: normal lung versus injured lung. J Intensive Care 2015;3:18. [Crossref] [PubMed]
- Negrini D, Moriondo A. Pleural function and lymphatics. Acta Physiol (Oxf) 2013;207:244-59. [Crossref] [PubMed]
- Negrini D, Moriondo A, Mukenge S. Transmural pressure during cardiogenic oscillations in rodent diaphragmatic lymphatic vessels. Lymphat Res Biol 2004;2:69-81. [Crossref] [PubMed]
- Moriondo A, Grimaldi A, Sciacca L, et al. Regional recruitment of rat diaphragmatic lymphatics in response to increased pleural or peritoneal fluid load. J Physiol 2007;579:835-47. [Crossref] [PubMed]
- Negrini D. Pulmonary microvascular pressure profile during development of hydrostatic edema. Microcirculation 1995;2:173-80. [Crossref] [PubMed]
- Yoshida T, Fujino Y, Amato MB, et al. Fifty Years of Research in ARDS. Spontaneous Breathing during Mechanical Ventilation. Risks, Mechanisms, and Management. Am J Respir Crit Care Med 2017;195:985-92. [Crossref] [PubMed]
- Frostell C, Blomqvist H, Hedenstierna G, et al. Thoracic and abdominal lymph drainage in relation to mechanical ventilation and PEEP. Acta Anaesthesiol Scand 1987;31:405-12. [Crossref] [PubMed]
- Maybauer DM, Talke PO, Westphal M, et al. Positive end-expiratory pressure ventilation increases extravascular lung water due to a decrease in lung lymph flow. Anaesth Intensive Care 2006;34:329-33. [PubMed]
- Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999;282:54-61. [Crossref] [PubMed]
- Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 1998;157:1721-5. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Coelho FR, Cavriani G, Soares AL, et al. Lymphatic-borne IL-1beta and the inducible isoform of nitric oxide synthase trigger the bronchial hyporesponsiveness after intestinal ischema/reperfusion in rats. Shock 2007;28:694-9. [PubMed]
- Cavriani G, Domingos HV, Oliveira-Filho RM, et al. Lymphatic thoracic duct ligation modulates the serum levels of IL-1beta and IL-10 after intestinal ischemia/reperfusion in rats with the involvement of tumor necrosis factor alpha and nitric oxide. Shock 2007;27:209-13. [Crossref] [PubMed]
- Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013;188:220-30. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Lattuada M, Hedenstierna G. Abdominal lymph flow in an endotoxin sepsis model: influence of spontaneous breathing and mechanical ventilation. Crit Care Med 2006;34:2792-8. [Crossref] [PubMed]


