Should we titrate positive end-expiratory pressure based on an end-expiratory transpulmonary pressure?
Introduction
Although there is general agreement that reductions of tidal volume, plateau pressure, driving pressure and driving power are key objectives in the protection of the injured lung (1), the best way of setting the end-expiratory pressure platform for tidal inflation [positive end-expiratory pressure (PEEP)] remains an issue of debate—as it has been for decades.
Adjusting PEEP levels simultaneously influences gas exchange, hemodynamics, and tissue stresses, but not necessarily all in the same direction toward benefit or harm (2). The extent to which each of these three responses occurs depends not only on the PEEP level and the other ventilation parameters, but also on the mechanical properties of the lung and chest wall to which it is applied and to some degree on body positioning (3).
Most would acknowledge that some minimally positive value of PEEP is appropriate for virtually all patients to compensate for the volume loss of recumbency and to avoid atelectasis. Some practitioners argue that the specific level of end expiratory pressure carries relatively little import once a modest minimum value is applied, while others point to the improved oxygenation, lower FiO2, and the (arguably) reduced risk of atelectraumatic lung injury [ventilator-induced lung injury (VILI)] that often accompanies higher PEEP values (4). What all caregivers understand, however, is that patients vary in their responses to PEEP.
As awareness grew of the potential for ventilating stresses to exacerbate lung injury, so did our understanding of volutrauma (overstretch) and atelectrauma (in part due to injurious tidal collapse and re-opening)—two processes that often move in competing directions as PEEP increases within the mechanically heterogeneous lung (5). This competition arises in major part from the gradient of transpulmonary pressure that exists to some extent in every position, due to gravitational forces and lung-chest wall shape disparities (6). These gradients are attenuated by a shift from supine to prone position (7). PEEP’s primary benefits pertain to prevention of de-recruitment and maintenance or expansion of the capacity of the ‘baby lung’ to accept the selected tidal volume. Conversely, PEEP’s potential for volutrauma parallels the accompanying rise of trans-pulmonary pressure it generates. This unavoidable competition between recruitment and overdistention must be understood to logically formulate an approach to optimizing PEEP selection. For a given tidal volume, the objectives of improved gas exchange and lung protection are often achieved with least hemodynamic compromise when compliance is best and driving pressure lowest (8).
How ‘open’ should we target the lung to be?
Advocates of the ‘open lung’ approach agree in principle but vary with recommendations regarding the aggressiveness with which establishing an ‘open lung’ should be pursued (9). The process of lung unit opening persists to some limited degree (especially in dependent zones) as volume increases from functional residual capacity (FRC) to total lung capacity (TLC) (10). Additional recruitment wanes and overdistention increases monotonically as transpulmonary pressure rises (6). Moreover, stress focuses at the junctions of units that inflate and tissues that remain unopened as mean airway pressure is raised (11). These junctional sites predominate in the mid-zones and more dependent sectors of the supine lung, but are not confined to them (12). At the bedside, the impetus to attempt further lung opening is often dictated by adequacy or inadequacy of gas exchange and constrained by hemodynamic tolerance.
In modern practice, certain non-conventional implementations of ventilation support prioritize an open lung and minimized tidal driving pressure. High frequency oscillation (HFO) and airway pressure release ventilation (APRV) are good examples of that ‘open lung’ bias. But when using traditional modes such as volume-controlled ventilation (VCV) and pressure-controlled ventilation (PCV) assist-control, considerable uncertainty remains as to whether striving for a more ‘fully open’ lung is advisable. A fundamental argument concerns whether PEEP should be geared to minimize driving pressure associated with the selected tidal volume or to establish a positive end-expiratory transpulmonary pressure (13). The latter objective tends to settle on a higher PEEP value (14). Concern for using that lung opening approach is well founded, however, as the hazard to benefit ratio regarding VILI and hemodynamics rises disproportionately with the magnitude of the applied peak, mean and driving airway pressures. On the other hand, if elevating mean airway pressure also improves oxygen exchange, it may allow reduction of potentially toxic levels of FiO2 (15).
Titrating techniques for setting PEEP
There can be little doubt that the pressure across the alveolar structures of the lung, the trans-pulmonary pressure, is more relevant than the raw airway pressure in determining tissue stresses and strains of interest. For want of a better measurable estimate of the pressures surrounding the alveolus, the pleural pressure has been used as the relevant ‘external’ pressure. But pleural and transpulmonary pressures vary topographically throughout the lung, with collapse of individual lung units invariably prevailing in gravitationally dependent areas (16). To assess transpulmonary pressure at the bedside, clinicians are currently obligated to use the esophageal balloon catheter to sense pleural pressure (17) (Figure 1). This well validated device reflects average global changes in pleural pressure rather well (18). Although Pes does not directly measure the pleural pressure at remote sites, it appears from the results of a recent study that Pes also reliably indicates the absolute pleural pressures that exist across its entire isogravitational (horizontal) plane (19). In an influential paper Talmor and colleagues (20) reported benefit to several important intermediate clinical outcomes when sufficient PEEP was used to force the local end-expiratory transpulmonary pressure (PTM) to transition from negative (indicating end-expiratory closure or collapse) to positive (indicating sustained patency in that zone). This transition point of PTM polarity change required application of rather high values of PEEP and mean airway pressure in comparison to other widely used titration methods, such as the traditional oxygenation-guided incremental approach or decremental adjustment following a recruitment maneuver (RM-Dec) (21).
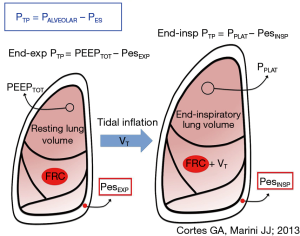
The physiologic rationale for the RM-Dec method is well grounded. The unstable lung units of acute respiratory distress syndrome (ARDS) require a higher pressure to recruit than that which maintains their patency (22). Although some lung units may require airway pressures in excess of 45 cmH2O to open, most remain so at 15 cmH2O PEEP or less (10). Both the pressure amplitude and duration of application influence lung unit opening; however, the majority of recruitment possible at a given recruiting pressure is completed within the first 10 seconds of its application, whereas the risk for hemodynamic compromise rises with passing time (23,24). Species vary with regard to the specific elastance of their healthy lungs; therefore, a pressure of 5 cmH2O in a rat might induce similar tissue stress and strain as 10 cmH2O in a pig or even higher pressures in a human (25). When the chest wall is abnormally stiff, as in morbid obesity, maintenance of dependent airway patency at end-expiration in a healthy lung may require PEEP values that are surprisingly high (26). Surfactant-depleted and diseased lung units require higher PEEP to maintain open status than their healthy counterparts (27).
Recruitability of acutely injured lung tissue varies with nature of the injury, stage and severity. After the first days of ARDS, perhaps only 10–20% of lung units that appear airless by computed tomography performed at low PEEP are potentially recruitable by 45 cmH2O (28), and those most refractory to opening and staying patent concentrate in dependent zones (29). Once opened, these diseased and inflamed units are imperfectly functional, inclined to early closure, and particularly susceptible to damaging excursions of driving pressure and concentrated power (30). Opening and closure (atelectrauma) and junctional stress amplification are not entirely eliminated at higher PEEP but may simply occur in fewer units. Those junctional units that remain closed are placed by the raised distending force at even higher risk for adverse consequences by subsequent tidal cycling (31) (Figure 1). Said differently, raising PEEP invariably increases mean airway, transpulmonary, and pleural pressures, boosting global and hemodynamic stresses as well as the strains experienced by interfacial tissues that remain unrecruited. Therefore, while refractory atelectasis may be partially reversible, the cost can prove high.
The place of a recruiting maneuver (RM) in PEEP selection
Current evidence indicates that the key ventilating parameter of the individual tidal cycle that influences clinical outcome is the driving pressure, i.e., the quotient of tidal volume and respiratory system compliance (32). In fact, minimizing that variable was shown in the seminal paper of Suter, Isenberg, and Fairley to hold promise for identifying best physiologic PEEP in ARDS by incremental titration (8). Although those early investigators did not use ‘low’ and ‘lung protective’ tidal volumes, the highest respiratory system compliance (least driving pressure) optimized the targeted variables of dead space fraction, oxygen saturation and O2 delivery (8). In later years, RM techniques were perfected, informing a decremental approach to setting PEEP after a stepwise (‘staircase’) RM to TLC (14) (Figure 2). Higher PEEP levels used in a well-conducted multi-center clinical trial improved mortality rate and intermediate outcomes relative to the comparison group (33). The staircase type of RM allows continued ventilation during its application and imposes a similar peak pressure but lower mean airway pressure than does the traditional RM characterized by application of high sustained airway pressure. The latter is exemplified by the 40 cmH2O for 40 seconds (‘40-40’) technique (34). The maximum RM airway pressure (and PEEP) that is needed for effectiveness or tolerance differs for patients with normally flexible and stiffer chest walls.
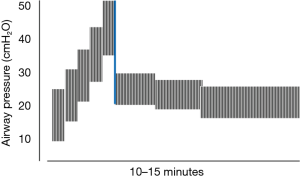
How exactly the RM is conducted, i.e., the amplitude, pattern, and duration of the recruiting pressure, may be important both to efficacy and hazard. The nature of the RM—maximal pressure achieved rapidly or slowly, for example (35), influences its relative safety. Hemodynamic disturbances during the RM, typically identified by a falling blood pressure, are greater with high sustained pressures (36) and occur more predictably in patients whose lungs are relatively refractory to opening (37,38). These hemodynamic problems recede quickly after release of the recruiting pressure. Sustained high transpulmonary pressures have been shown to be potentially tissue damaging in both pre-injured rodents (39) and healthy pigs (40). The durability of benefit from the RM also depends on the tidal ventilation pattern (PEEP and tidal volume) that follows its application (24).
Whether oxygenation or mechanics should be used to guide PEEP selection has been actively debated. The tabular guidance approach to PEEP and FiO2 selection used by the ARDSNet has served in original and modified forms in subsequent studies and is now employed in many ICUs for everyday clinical decision making (41). However, while oxygenation efficiency is one important axis of the set of targeted variables relevant to PEEP, oxygenation is not the most logical one to use if the prime concern is lung protection, as PaO2 is heavily influenced by re-distribution of pulmonary blood flows, systemic venous O2 saturation, and hypoxic pulmonary vasoconstriction. True optimization for lung protection would seem more logically served by other ‘open lung’ approaches: the stress index, the post-RM decremental method or the end-expiratory transpulmonary pressure conversion to positivity (PTM polarity transition) (13). Based on presently available experimental and clinical evidence (19,20), the latter might seem a highly rational option. Nonetheless, it may be an inferior choice for reasons explained in the following discussion.
Rationale and limitations of the Pes polarity shift for setting PEEP
Measuring end-expiratory transpulmonary pressure (PEEP minus end-expiratory pleural pressure) relies on the esophageal pressure recording, as this is the only clinically feasible way of directly sampling pleural pressure. Unfortunately, Pes does not accurately represent absolute pressures elsewhere in the chest that lie above or below the catheter’s own horizontal plane. Moreover, in the supine horizontal position, the esophagus is situated in the mid to dependent thorax. The catheter balloon lies beneath the heart and mediastinum, and these anatomic structures have been suspected by many—even PTM advocates—to impose a local weight on the supporting lung (42) and esophagus in the supine position that adds artifactually to the transmitted pleural pressure value. End-expiratory transpulmonary pressure calculations using the difference between PEEP and Pes, therefore, have been supplemented by 2–5 cmH2O in an attempt to account for this superimposed weight that, in theory, leads to an underestimate of relevant alveolar stretch (13,20). The need to ‘correct’ for this local weight by adjusting PEEP upward in determining actual lung stress may be ill-founded, however; recently published data demonstrate both in pigs and in human cadavers that the absolute value of the Pes not only reflects its own local environment but also that of isogravitational pleural pressures at the lungs’ lateral surfaces remote from heart and mediastinum (19).
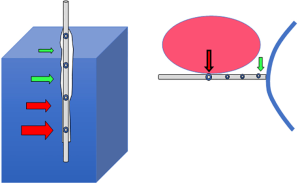
One reason for that surprising equivalence may be that when appropriately filled, the 10 cm-long flaccid balloon tends to transmit to the catheter lumen and transducer the least local pressure along its entire length (43) (Figure 3). The most caudal portions of the balloon (and the catheter holes that communicate with it) are situated between the cardiac apex and the diaphragmatic crux. In other words, unless the diaphragm dome rides exceptionally high in the chest, there may be little to no artifact that relates to superimposed weight from mediastinum or abdomen. An impressive laboratory and clinical investigation of PTP that targeted healthy obese subjects employed Pes, computed tomographic imaging, and direct hemodynamic measurements to show that in RM-Dec titration, PTP polarity shift is an effective guide to hemodynamically well-tolerated lung opening (26). Good correspondence between Pes and lung surface pressure, does not mean, however, that that the raw (PEEP-Pes) calculation is directly relevant to the more dorsal lung units that lie beneath the catheter’s own horizontal plane. Therefore, even when the transpulmonary PEEP turns positive, collapsed alveoli could exist that could potentially open under PEEP values that are even higher. The Pes-based method for setting PEEP, the ‘PTM polarity’ transition, therefore, does not keep the most dependent lung regions fully ‘open’.
Another method for setting PEEP using the Pes and PTM measurements is less direct and may give a better indication of the highest stress to which open units are exposed at end-inspiration (44). Elastances of the chest wall and lung add serially to equal that of the respiratory system. By assessing the elastance of the passive chest wall using Pes responses to tidal volume increments and measuring elastance of the integrated respiratory system using airway pressures [(Pplat-PEEP)/VT], an estimate of the missing unmeasured variable of interest—lung elastance—can be computed. From that latter parameter, the maximum inflation stretching pressure can be inferred. Unfortunately, this elastance-based technique and the directly measured ‘PTM polarity’ transition pressure—though both making use of the Pes—do not assign the same values to ‘optimal’ PEEP (44).
Is the Pes really needed to set PEEP?
The need to estimate PEEPTM at all has been questioned on the basis that both in patients with ARDS and those with massive obesity (45), the compliance of the chest wall does not markedly alter the key mechanical properties of the integrated respiratory system (CRS). In the former circumstance the injured ‘baby lung’ is the predominating factor determining the CRS, whereas in the latter, the heavy chest wall is said to act as a fixed weight that displaces the Pes-Volume curve rightward but does not alter its slope (46). Although this contention remains highly debatable for ICU patients, it is generally true that the slope of the airway pressure-volume inflation curve for the respiratory system tends to parallel that of the lung. The ‘stress index’, a curve-shaping parameter obtained during inflation with constant inspiratory flow, draws upon that premise and weighs the balance between overdistention and recruitment during passive tidal breathing (47). Its relative ease has gained the stress index popularity as a practical alternative method for setting PEEP for passively ventilated patients the clinical setting. The stress index is attractive in its recognition of the inherent competition between further recruitment and overdistention in response to rising PEEP that persists to TLC using (10). Convincing comparative studies showing its superiority however, remain lacking.
Comparing post-recruitment decremental and transpulmonary polarity transition methods
Several experimental studies have confirmed that once the mean airway pressure has been driven high enough by PEEP to tip the scales toward overdistention, both healthy and injured tissues sustain further damage. That injury threshold may be as low as 7 cmH2O for the lungs of healthy pigs and lower still in those of the comparatively fragile rat (25). In any comparison between decremental and ‘artifact-adjusted Pes’ titration of PEEP, it is worth considering that the latter trends toward a higher value in comparison to the former. This bias implies that alveoli in less dependent lung zones tend toward overstretch when PEEP is Pes-set.
Although each method has a good physiologic justification, no study has clearly demonstrated the superiority of the stress index, RM-Dec, or PTM polarity transition methods in a head-to-head comparison (48). Of these, the RM-Dec method has perhaps the best scientific rationale. Unfortunately, it also is the least well standardized and therefore most subject in practice to implementation variations. It may not be surprising, therefore, that this technique has been reported to offer both strikingly positive (33) and strikingly negative (49) clinical impact. A recently published and highly influential RCT that used RM-Dec, the Alveolar Recruitment for ARDS (‘ART’) Trial, was designed with the intent to show the superiority of this technique for ‘opening’ the lung (49). Disappointingly, however, just the opposite was implied by the results. The ART trial has been strongly and cogently criticized, however, for its flawed design and execution (50). Given the deficiencies of the ART study, many informed practitioners continue to utilize a less aggressive RM-Dec variant in their clinical practices to set PEEP for moderately to severely ill patients with ARDS.
Summary
Arguments continue to swirl regarding the need for and best method of PEEP titration. In many centers, the ARDSNet table provides a convenient guide that helps assure consistency across a range of caregivers with varying skill sets and experience. Unfortunately, despite its pragmatic appeal, tabulated guidance of this type lacks a solid scientific underpinning. Assuming that a ‘safe’ driving pressure has been selected, that the slope can be precisely measured, that passive conditions apply, and that an abnormal chest wall or pleural effusion is not a major factor (51), the stress index helps identify the PEEP value associated with best compliance and presumably strikes a PEEP trade-off that applies acceptable tissue and hemodynamic stresses. An appropriately conducted RM-Dec that uses modest peak pressures, relatively small PEEP increments and appropriate timing intervals is currently the most logical and therefore an attractive option, particularly when the Pes is used to calculate transpulmonary driving pressures relevant to the lung. Finally, the setting of PEEP by the Pes-guided end-expiratory ‘polarity transition’ point is limited by its tendency to encourage PEEP levels that are higher than the RM-Dec. These are likely unnecessary, and therefore are associated with a relatively high hazard to benefit ratio. We currently await publication of results from the EPVent2 trial (52) testing the merit of the PTM polarity transition approach. Unless and until the polarity transition point methodology proves its merit, there would seem to be better alternatives available.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Toung TJ, Saharia P, Mitzner WA, et al. The beneficial and harmful effects of positive end expiratory pressure. Surg Gynecol Obstet 1978;147:518-24. [PubMed]
- Keenan JC, Cortes-Puentes GA, Zhang L, et al. PEEP titration: The effect of prone position and abdominal pressure in an ARDS model. Intensive Care Med Exp 2018;6:3. [Crossref] [PubMed]
- Lachmann B. Open up the lung and keep the lung open. Intensive Care Med 1992;18:319-21. [Crossref] [PubMed]
- Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998;157:294-323. [Crossref] [PubMed]
- Pelosi P, Goldner M, McKibben A, et al. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 2001;164:122-30. [Crossref] [PubMed]
- Gattinoni L, Taccone P, Carlesso E, et al. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med 2013;188:1286-93. [Crossref] [PubMed]
- Suter PM, Fairley B, Isenberg MD. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med 1975;292:284-9. [Crossref] [PubMed]
- Sahetya SK, Brower RG. Lung Recruitment and Titrated PEEP in Moderate to Severe ARDS: Is the Door Closing on the Open Lung? JAMA 2017;318:1327-9. [Crossref] [PubMed]
- Crotti S, Mascheroni D, Caironi P, et al. Recruitment and derecruitment during acute respiratory failure: a clinical study. Am J Respir Crit Care Med 2001;164:131-40. [Crossref] [PubMed]
- Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970;28:596-608. [Crossref] [PubMed]
- Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2007;175:160-6. [Crossref] [PubMed]
- Loring SH, O’Donnell CR, Behazin N, et al. Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol (1985) 2010;108:515-22. [PubMed]
- Borges JB, Okamoto VN, Matos GF, et al. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;174:268-78. [Crossref] [PubMed]
- Carraway MS, Piantadosi CA. Oxygen toxicity. Respir Care Clin N Am 1999;5:265-95. [PubMed]
- Pesenti A, Musch G, Lichtenstein D, et al. Imaging in acute respiratory distress syndrome. Intensive Care Med 2016;42:686-98. [Crossref] [PubMed]
- Mauri T, Yoshida T, Bellani G, et al. PLeUral pressure working Group (PLUG—Acute Respiratory Failure section of the European Society of Intensive Care Medicine). Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 2016;42:1360-73. [Crossref] [PubMed]
- Akoumianaki E, Maggiore SM, Valenza F, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014;189:520-31. [Crossref] [PubMed]
- Yoshida T, Amato MB, Grieco DL, et al. Esophageal Manometry and Regional Transpulmonary Pressure in Lung Injury. Am J Respir Crit Care Med 2018;197:1018-26. [Crossref] [PubMed]
- Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095-104. [Crossref] [PubMed]
- Marini JJ. How best to recruit the injured lung? Crit Care 2008;12:159. [Crossref] [PubMed]
- Faffe DS, Zin WA. Lung parenchymal mechanics in health and disease. Physiol Rev 2009;89:759-75. [Crossref] [PubMed]
- Arnal JM, Paquet J, Wysocki M, et al. Optimal duration of a sustained inflation recruitment maneuver in ARDS patients. Intensive Care Med 2011;37:1588-94. [Crossref] [PubMed]
- Lim SC, Adams AB, Simonson DA, et al. Intercomparison of recruitment maneuver efficacy in three models of acute lung injury. Crit Care Med 2004;32:2371-77. [Crossref] [PubMed]
- Caironi P, Langer T, Carlesso E, et al. Time to generate ventilator-induced lung injury among mammals with healthy lungs: a unifying hypothesis. Intensive Care Med 2011;37:1913-20. [Crossref] [PubMed]
- Fumagalli J, Berra L, Zhang C, et al. Transpulmonary Pressure Describes Lung Morphology During Decremental Positive End-Expiratory Pressure Trials in Obesity. Crit Care Med 2017;45:1374-81. [Crossref] [PubMed]
- Albert RK. The role of ventilation-induced surfactant dysfunction and atelectasis in causing acute respiratory distress syndrome. Am J Respir Crit Care Med 2012;185:702-8. [Crossref] [PubMed]
- Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006;354:1775-86. [Crossref] [PubMed]
- Gattinoni L, Marini JJ, Pesenti A, et al. The “baby lung” became an adult. Intensive Care Med 2016;42:663-73. [Crossref] [PubMed]
- Marini JJ, Gattinoni L. Energetics and the root mechanical cause of VILI. Anesthesiology 2018;128:1062-4. [Crossref] [PubMed]
- Gattinoni L, Tonetti T, Cressoni M, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med 2016;42:1567-75. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Villar J, Kacmarek RM, Pérez-Méndez L, et al. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006;34:1311-8. [Crossref] [PubMed]
- Marini JJ. Recruitment maneuvers to achieve an “open lung”--whether and how? Crit Care Med 2001;29:1647-8. [Crossref] [PubMed]
- Silva PL, Moraes L, Santos RS, et al. Impact of pressure profile and duration of recruitment maneuvers on morphofunctional and biochemical variables in experimental lung injury. Crit Care Med 2011;39:1074-81. [Crossref] [PubMed]
- Lim SC, Adams AB, Simonson DA, et al. Transient hemodynamic effects of recruitment maneuvers in three experimental models of acute lung injury. Crit Care Med 2004;32:2378-84. [Crossref] [PubMed]
- Grasso S, Mascia L, Del Turco M, et al. Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology 2002;96:795-802. [Crossref] [PubMed]
- Mercado P, Maizel J, Kontar L, et al. Moderate and Severe Acute Respiratory Distress Syndrome: Hemodynamic and Cardiac Effects of an Open Lung Strategy With Recruitment Maneuver Analyzed Using Echocardiography. Crit Care Med 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Santos RS, Moraes L, Samary CS, et al. Fast Versus Slow Recruitment Maneuver at Different Degrees of Acute Lung Inflammation Induced by Experimental Sepsis. Anesth Analg 2016;122:1089-100. [Crossref] [PubMed]
- Collino F, Rapetti F, Vasques F, et al. Positive end-expiratory pressure and mechanical power. Anesthesiology 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. [Crossref] [PubMed]
- Albert RK, Hubmayr RD. The prone position eliminates compression of the lungs by the heart. Am J Respir Crit Care Med 2000;161:1660-5. [Crossref] [PubMed]
- Higgs BD, Behrakis PK, Bevan DR, et al. Measurement of pleural pressure with esophageal balloon in anesthetized humans. Anesthesiology 1983;59:340-3. [Crossref] [PubMed]
- Chiumello D, Cressoni M, Colombo A, et al. The assessment of transpulmonary pressure in mechanically ventilated ARDS patients. Intensive Care Med 2014;40:1670-8. [Crossref] [PubMed]
- Behazin N, Jones SB, Cohen RI, et al. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol 2010;108:212-8. [Crossref] [PubMed]
- Pelosi P, Luecke T, Rocco PR. Chest wall mechanics and abdominal pressure during general anaesthesia in normal and obese individuals and in acute lung injury. Curr Opin Crit Care 2011;17:72-9. [Crossref] [PubMed]
- Terragni P, Bussone G, Mascia L. Dynamic airway pressure-time curve profile (Stress Index): a systematic review. Minerva Anestesiol 2016;82:58-68. [PubMed]
- Caramez MP, Kacmarek RM, Helmy M, et al. A comparison of methods to identify open-lung PEEP. Intensive Care Med 2009;35:740-7. [Crossref] [PubMed]
- Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. [Crossref] [PubMed]
- Villar J, Suárez-Sipmann F, Kacmarek RM. Should the ART trial change our practice? J Thorac Dis 2017;9:4871-7. [Crossref] [PubMed]
- Formenti P, Graf J, Santos A, et al. Non-pulmonary factors strongly influence the stress index. Intensive Care Med 2011;37:594-600. [Crossref] [PubMed]
- Fish E, Novack V, Banner-Goodspeed VM, et al. The Esophageal Pressure-Guided Ventilation 2 (EPVent2) trial protocol: a multicentre, randomised clinical trial of mechanical ventilation guided by transpulmonary pressure. BMJ Open 2014;4. [Crossref] [PubMed]


